Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and capsule filling.
Manufacturing DPIs: IMA Adapta® with Boehringer Ingelheim Spiriva®
Summary
When producing pharmaceutical products in DPI form, industrial manufacturing aspects must be considered together with optimization of the process, as well as to achieve a reliable control method. Precise micro-dosing, gravimetric weight control, containment measures and ease of device assembly are typical issues which could be faced. IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and capsule filling. Direct weight control performed in line on each single capsule, both before and after filling. Absence of mechanical powder compression for improved airway intake. Accurate micro-dosing and automatic feedback and adjustment. This presentation investigates optimal process parameters for low-dose DPIs achieved by the dosator technology. The study proves that a major advantage of using this technology for processing DPIs is that the dosators can be accurately adjusted without any need to compress or aspirate the powder. Maintaining the free-flowing properties of the dispended powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced, thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.
Key message
The key to achieve optimal filling and control of low-dose Dry Powder for Inhalers is combining the dosator technology and the direct gravimetric net weight control. This is now available on an industrial production scale capsule filler.
Introduction
In 1948, the first commercial dry powder inhalation device was launched on the market. This first technology seems archaic by today’s standards: a deep inward breath would cause a ball to strike a cartridge containing powder and shake the powder into the airstream. Since then, changes in the drug delivery market and regulatory pressures have driven innovation of DPIs forward. It is estimated by the WHO that, worldwide, some 300 million people suffer from asthma and 240 million people suffer from chronic obstructive disease (COPD). DPIs represent 50% of the total asthma/COPD market by value worldwide.
The latest patient-focused studies using DPIs indicated that the expectations regarding this technology have evolved. Patients and pneumologists are now increasingly focusing on convenience and ease of use, favouring a compact design. Indeed, DPIs have shown great promise in their ability to deliver drugs reliably and effectively, and novel designs can ensure that future cost, compliance and safety challenges are overcome. Some of the performance characteristics essential to DPIs are related to dose delivery, fine particle fraction content and performance levels at varying airflows. These characteristics can differ from one powder formulation to another, and some fine tuning of either device or formulation or a combination of both may be necessary to achieve optimal performance. Micro-dosing DPIs takes this challenge to extremes. IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and assembly.
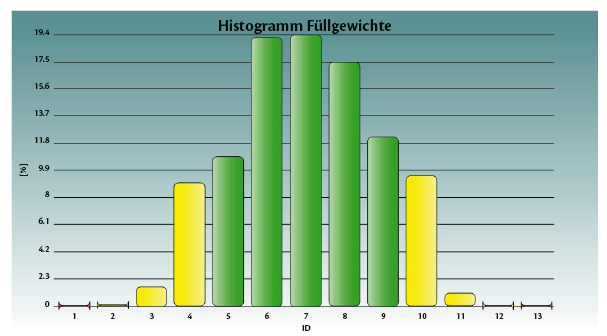
Graph 1: weighing statistics for the Total batch (T) and for each dosator individually.
Case study: cooperation of two among the world-wide leading companies in the DPIs to achieve the best solution for Spiriva® reliable and effective production.
Aim of the study
The aim of the study was to explore the best process parameters to achieve the 5.5 mg dose of a powder mix including Tiotropium and a Lactose carrier. Boehringer Ingelheim initially carried out the process on various different capsule filler types and then optimized it thanks to IMA capsule filling machine model Adapta 100 with 100% gravimetric net weight control.
Materials
Components of the case study are:
A) Blend of Lactose based carrier and Tiotropium API, which is available on the market with the name of Spiriva® by Boehringer Ingelheim GmbH & C. (Germany).
B) Adapta 100 (IMA, Italy) an industrial scale production capsule filler implementing dosator needle technology together with gravimetric 100% net fill weight checking system with scales resolution < 0.01 mg (ISO 22514-7).
Methods
The first steps of the study were conducted by Boehringer Ingelheim alone.
Several capsule filling machines were tested, with several processing technologies (continuous, intermittent motion) several dosing systems (dosator needle, vacuumed drum filler), several systems for mass control (capacitive sensors, elastomers for gravimetric weighing).
The processing technology do not have a direct impact on performance, unless needed for other features such as gravimetric weighing.
The dosator needle was preferred when compared with drum filler for the following reasons:
- Dosator fills the dosing chamber effectively. The drum filler relies on the force of gravity to fill the chamber (ineffective and difficult to control) and vacuum (irregular since air follows preferential routes inside the powder, creating a “rat hole” and besides it the membrane clogs soon).
- Dosator holds micronized powders in the chamber thanks to their cohesivity and very small dosator diameter. In addition, IMA applies a patented syringe effect. Vacuumed drum filler relies on the vacuum to hold the powder in the chamber, thin particles are aspirated, and this generates high losses of product and frequent stops.
- Average weight centered on the target weight. The chamber height of each dosator can be accurately set and individually adjusted.
Analysis of the process
After the pre-runs the process was analyzed and studied in deep detail.
IMA and Boehringer Ingelheim put in place high qualified resources specialized in mechanical design, statistics and mathematics analysis and software development.
Several tests were performed at IMA factory on Adapta with 100% gravimetric net weight control. The below process analysis loop was followed (Graph 2).
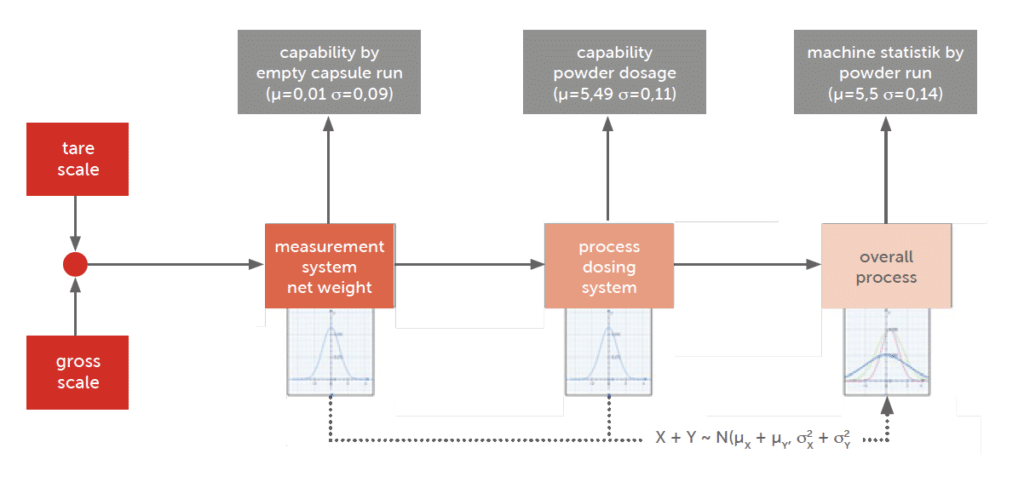
Graph 2: process analysis loop.
The pre-runs showed that the Adapta machine was fully capable of handling Spiriva® capsules and that the dosing by dosators was perfectly achieving the needs for the product. Boheringer Ingelheim worked together with IMA to find the right dynamic parameters and optimize the closed loop control. The weighing system was optimized. Several tests were performed when no powder was dosed.
The “ideal” system should result in a net weight equal to zero, since the capsule is empty. The Standard Deviation represents the possible errors: scale accuracy, air flows, for example due to the vacuum opening, vibration, etc.
The timing for the weight sampling was changed and the calibration weight was set to 100 mg (tolerance class E2). Table 1 shows the results achieved.
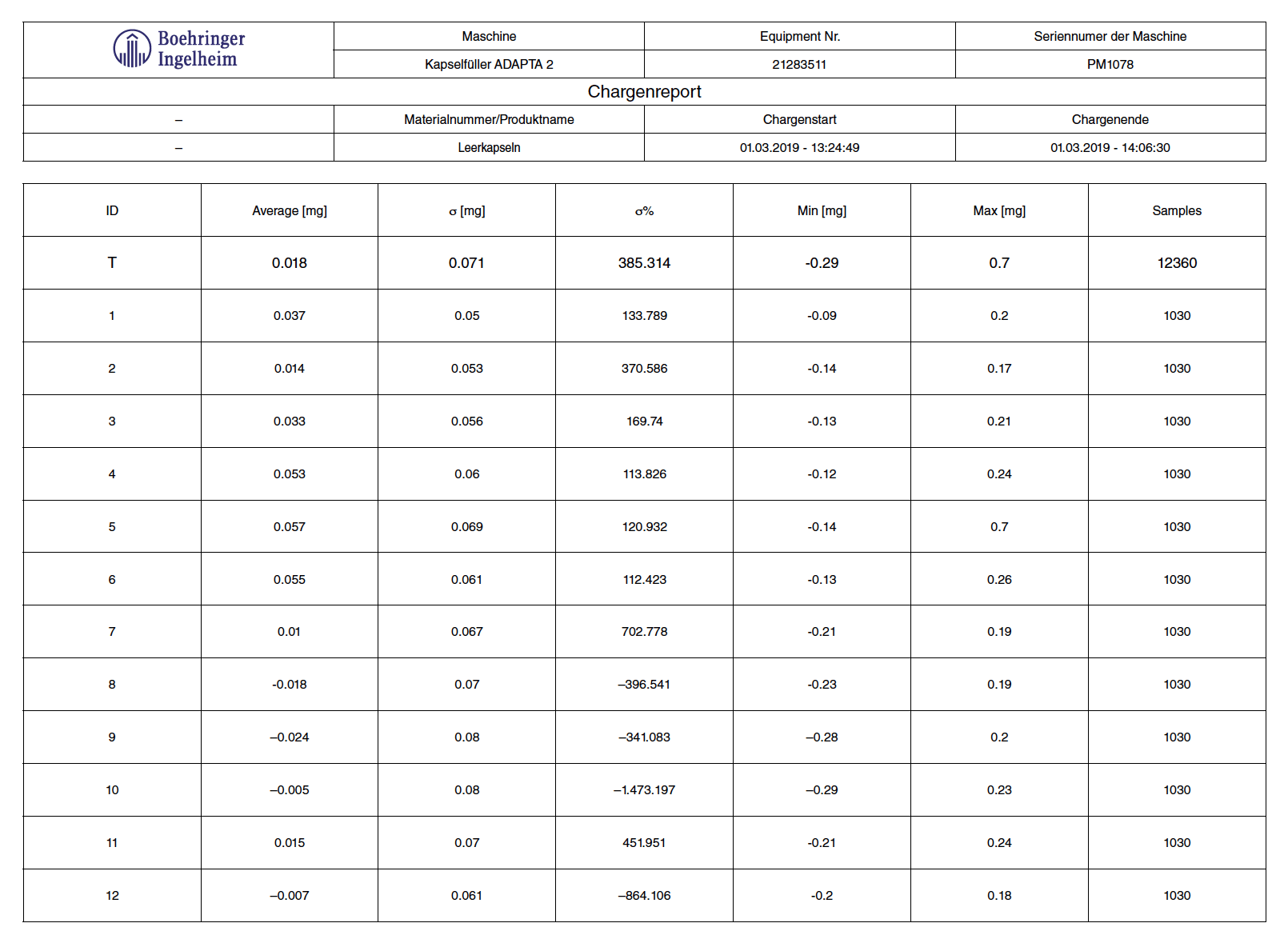
Table 1: test with empty capsules on Adapta with 100% gravimetric net weight control.
Process requirements
- Capsule size 3
- Powder for inhalation
- Net fill weight 5.5 mg +-10% ⇒ LSL = 4.95 mg, USL= 6.05 mg
- 100% control for filling
- Smooth capsule run
- Easy to use: fast and setting up / cleaning / operate
- Production data accessible
Technical criteria
- Machine speed
- Machine size
- Machine kind (intermitted pulsed/continuous run)
- Filling technology (dosator needle, sliding chamber, vacuumed drum…)
- Capsule handling: opening/move (body, cap) / close
- Machine complexity (easy to run)
- Set up and disassemble speed
- GMP design (surfaces/materials/easy to clean/no dead room…)
- Measurement system (principle/stability/influences…)
- Sold machines in the market (support, developed to use…)
- Costs (invest, maintenance, media…)
Experimental part
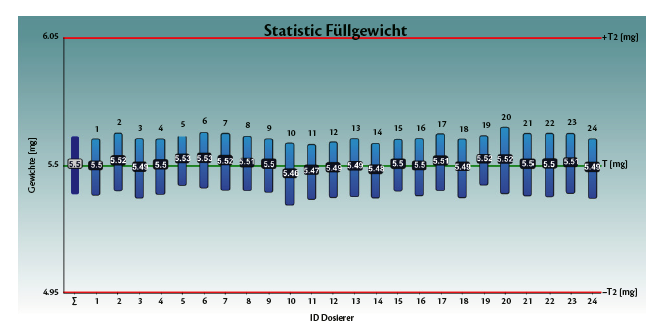
Tables 1 and 2, and graphs 3 and 4 show the achieved fill weight for the total batch (“T”) and for each dosator individually.
Perfectly centered and on target (µ= 5.5 mg and Bias= 0.00 mg).
Results
After several sessions of trials, machine parts optimization, deep analysis of the measuring system and its synchronization with capsule filling process, Adapta with 100% gravimetric net weight control was able to perfectly match process capability
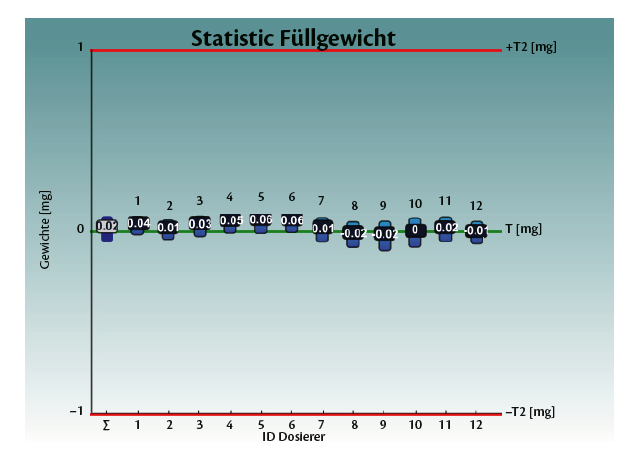
Graph 3: test with empty capsules on Adapta 100 with 100% gravimetric net weight control.
requirements with very low target dosage. And in particular Adapta is able to incorporate:
A very accurate and reliable system for auto-diagnosis, able to achieve:
- Standard deviation σ < 0.1 mg
- Bias -0.03 to + 0,03
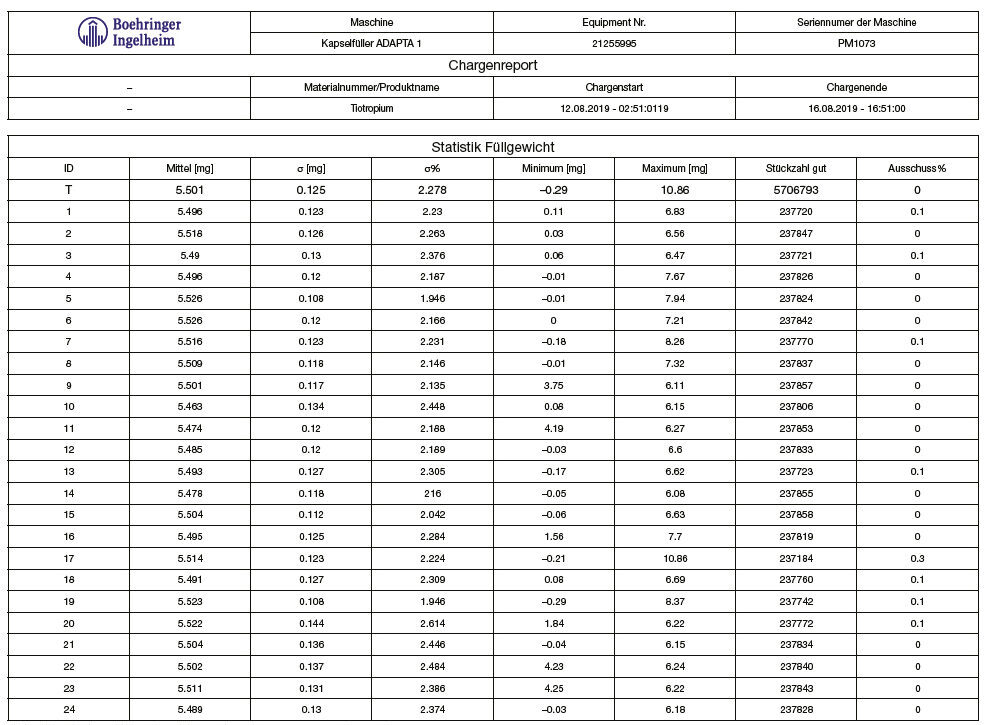
Table 2: Spiriva®, achieved fill weight on Adapta with 100% gravimetric net weight control.
Graph 4: Spiriva®, achieved fill weight on Adapta with 100% gravimetric net weight control.
• Standard deviation σ = 0.13 to – 0.16 mg
• Totally centered on average, with Bias 5.5 mg +- 0.05 mg
Discussion and conclusion
As proven by this study, a major advantage of using the dosator technology for processing low-dose Dry Powder Inhalers is that the system can dose very small amounts of powders into capsule. This powder dosing technology does not require powder compaction to transfer the powder to the capsule. This ensures that the powder within the capsule is less likely to form aggregates and is maintained as a free-flowing powder. Maintaining the free-flowing properties of the dispended powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced, thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.
References
[1] Edwards D., Applications of capsule dosing techniques for use in dry powder inhalers, “Therapeutic Delivery”, July 2010.
[2] Rogueda P., Take a deep breath. Inhalable drug delivery, “World Pharmaceuticals”, April 2016. [3] Williams G., The future of DPIs: Aligning Design with Market Demands, “Drug Development & Delivery”, December 2012.