
Tech Blog
Issues regarding cell & gene therapies, blood fractionation, environmental control, OEB and many other topics are dealt with by our staff on a regular basis.
The following are brief insights into how we at IMA Life endeavour to drive innovation within the industry and stay at the forefront, sharing our knowledge with the key players. More in-depth details can be obtained by discussing these topics with our experts.
Exceptional flexibility for high-performance fill-finish lines
Managing small batches in high containment situations
Providing a valid response to new GMP Annex 1 requirements
Gloveless automation technologies to deal with biological materials
How to balance sterility assurance with containment
Optimising the choice of HVAC solutions for isolated lines
Open RABS
Closed RABS
Isolators
Operation Cost Savings
Taking the strain out of sterile pharmaceutical and biotech production
Exceptional flexibility for high-performance fill-finish lines

IMA Life creates highly flexible, end-to-end, fill-finish solutions thanks to “combo” robotic fillers such as INJECTA, which can be adapted to vial or syringe production and are ready for bulk feeding or RTU packaging. The configuration of the pressure cascade and barrier technologies (isolators, closed RABS and open RABS) allows traditional production contexts to be managed, but may also enable set-up of high-containment production for high potent or biohazard products.
- robotic filling
- syringes, vials
- in bulk or RTU packaging
- configurable for high containment
Managing small batches in high containment situations
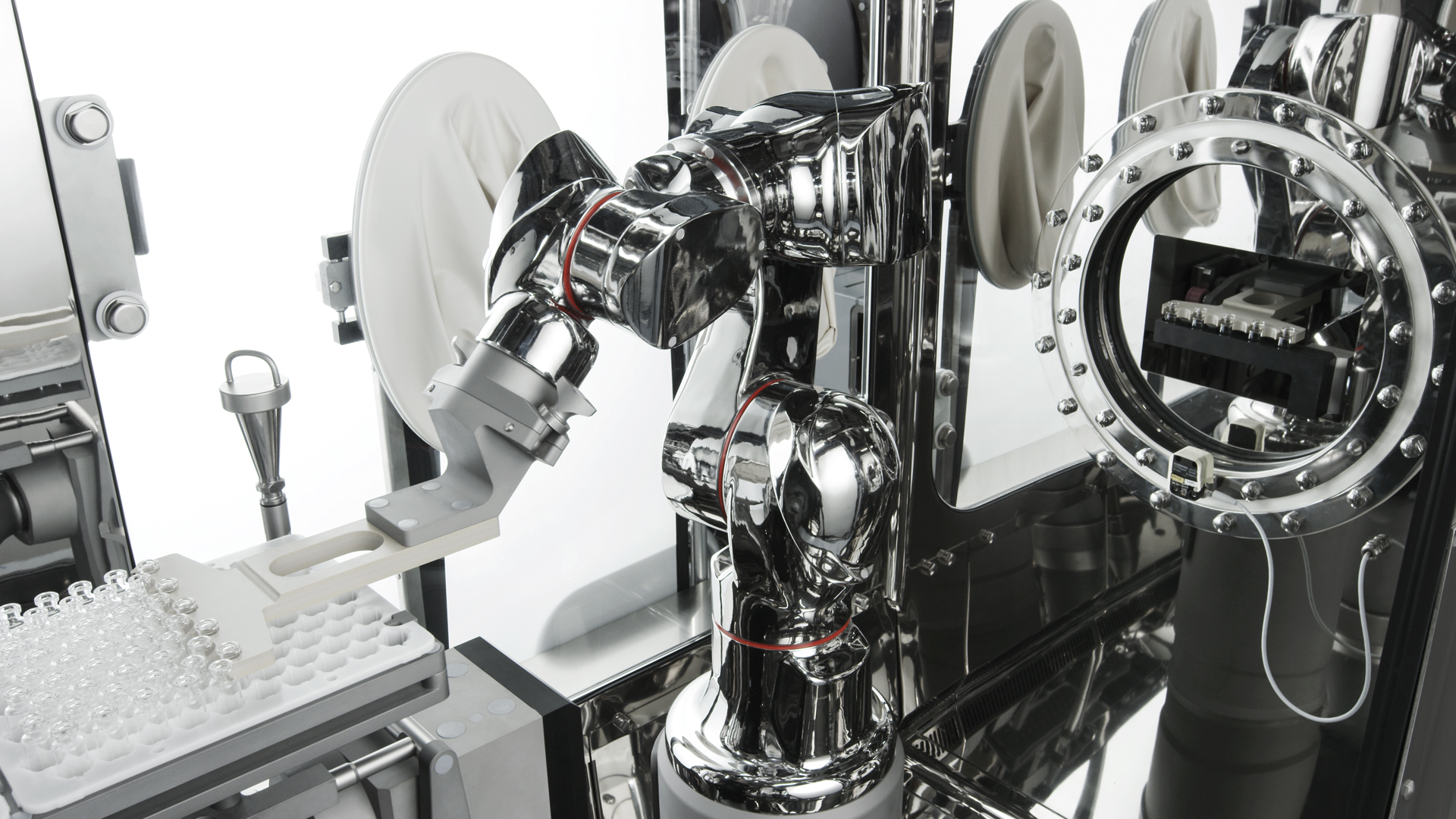
IMA Life designs and implements solutions with specific features for small batches, often for high value-added pharmaceutical specialities. These include filling strategies that minimise drug dispersion in the initial and final stages of production, so as to minimise vial waste. Isolators are equipped with ventilation that adapts temperature and humidity to product-specific requirements and capable of implementing high-toxic (OEB ≥5) and biological (BSL ≥2) containment strategies by configuring filtration, pressure cascade, maximising integrity (tightness) and equipping the isolator with VPHP-based automated deactivation systems and automated wash-in-place solutions to reduce residual risk at the end of production.
- small batch production
- high value-added products
- specialities for clinical or pre-clinical batches
- advanced biologics
Providing a valid response to new GMP Annex 1 requirements

For IMA Life, the requirements of the new GMP Annex 1 are the starting point for the design of automatic machines and barrier systems, both RABS and isolators. Unidirectional airflow dynamics within Grade A zones are assessed during the design phase using CFD methodologies, followed by on-site experimental tests. The goal is to maximise compliance with the first air requirement, and minimise sources of risk for critical processes in the aseptic zone. Operating procedures are defined to handle parts in direct and indirect contact with the product. The correct methodology for introducing critical material is determined. A hygienic and ergonomic design of the entire machine-isolator system is implemented, and a viable and non-viable monitoring strategy adapted to regulatory requirements is proposed.
- compliance with first air requirement
- management of parts in contact with the product
- hygienic and ergonomic design
- viable and non-viable monitoring
Gloveless automation technologies to deal with biological materials

IMA Life has developed innovative technologies for the field of advanced therapies and cell & gene therapy. In such contexts, the filling line must suit the special requirements of the product: i.e. the presence of biological material in suspension; very limited stability times; high sensitivity to oxidising residues such as VPHP; need for cryopreservation at the end of the fill-finish process. Such requirements exclude traditional fill-finish solutions. For this reason, IMA LIFE implements fully robotised strategies on INJECTA machines and has developed TILE-X, a highly innovative modular and automated solution, integrated in an aseptic, gloveless environment.
- biological material in suspension
- material stays stable for a limited time
- sensitivity to oxidising residues
- possible need for cryopreservation
How to balance sterility assurance with containment

Combining sterility assurance requirements with high-containment contexts often compromises production flexibility, generating a significant impact on cost and complexity. With its experience in high containment, for both powder and liquid products, IMA Life designs fill-finish lines that meet the highest containment requirements for high-potent or biohazard contexts without compromising sterility assurance criteria and complying with GMP Annex 1 requirements. Lines can be equipped with solutions to reduce the risk of contamination at the end of production, such as wash-in-place systems, VPHP-based automated deactivation and switching the dynamic pressure cascade during the capping phase. Specific ventilation configurations such as full one-pass isolators (without recirculation) and with single or double filtration stages are also possible.
- wash-in-place systems
- deactivation via H2O2
- dynamic pressure cascade
- recirculation-free ventilation and multiple filtration
Optimising the choice of HVAC solutions for isolated lines

IMA Life adapts the ventilation management for RABS and isolators to the needs of the production environment and the space offered by the clean room and technical area. Isolators can be configured to extract pre-treated air from the clean room or the customer’s HVAC systems, minimising the impact on layout and system costs. Alternatively, different types of HVAC are implemented, with specific temperature and humidity requirements, whether or not supplied by outside air. Having detailed control of the isolator and HVAC, both at the design and construction level, allows IMA LIFE to optimise the solution. Configurations with special requirements are also possible, such as Grade A isolators with internal atmosphere (100% nitrogen) or isolators supporting cold filling or cold loading processes (freeze dryer loading with cold plates).
- pre-treated air from the clean room
- purpose-built HVAC systems with specific requirements
- modular and configurable isolators
- isolators for cold filling/cold loading processes
Open RABS
Gloves must be positioned in order to allow the operator to perform all operations inside the machine, such as cleaning, loading of caps/stoppers, removal of vials, etc. Thereby, these operations can be done by operators without opening the protection walls.
The RABS can be PASSIVE or ACTIVE type.
PASSIVE means that the system is NOT equipped with dedicated ventilation, in which case the airflow inside the RABS should be generated externally. Under normal circumstances, the unidirectional flow required in the aseptic area is produced by fans and filters embedded in the false ceiling of the production room.
ACTIVE means that the system is equipped with an independent air ventilation system, in that case the unidirectional flow required in the aseptic area is generated by fans and filters that are parts of the RABS itself, then the airflow is partially independent from the airflow of the production room.
In both cases the area inside the RABS must be “A” GMP class, and the surrounding area must be classified as “B”.
Both are considered OPEN because the air used for the unidirectional flow is expelled into the production room, without any control or filtration. Open RABS are useful in maintaining aseptic conditions in the line, but they are useless with toxic or biohazardous products, since they cannot offer any type of protection for the operator and the environment.
Advantages:
- Aseptic condition improvement in the line
- Minimal impact on building layout and utilities
- Minor impact due to qualification activities
Weaknesses:
- Surrounding production area must be Grade B (with an Isolator, it can be downgraded to the less expensive Grade C)
- No operator protection, not suggested with high potent or biohazardous products.
- Humidity, temperature and differential pressure inside the O-RABS is dependent on the environmental production room conditions.
- It is impossible to recycle the air inside the barrier, saving HVAC energy consumption.
- No possibility of performing WIP cycles (Wash In Place)
- No possibility of performing automatic decontamination cycles (for example with vaporised Hydrogen Peroxide) but able to sustain a cleanroom VHP decontamination if requested.
Closed RABS
Normally, air leak tightness classification for these systems is not carried out using the ISO 10648-2 standard, which is usually applied for isolators, since different solutions, with very different prices, are available on the market.
Due to the lack of leak-tight certification these systems cannot not be used for highly toxic products.
Moreover, these systems must assure a Grade A environment, and the surrounding area must be classified as B.
Advantages:
- Medium impact on building layout and utilities
- Minor impact due to qualification activities. Humidity, temperature and pressure inside the Closed RABS can be controlled adopting a dedicated HVAC
- Possibility of recycling the air used inside, saving HVAC energy consumption.
Weaknesses:
- Surrounding production area must be Grade GMP B (with an Isolator, it can be downgraded to the less expensive Grade C)
- Limited operator protection, not suggested for high potent or biohazardous products
- No possibility of performing WIP cycles (Wash In Place)
- No possibility of performing automatic decontamination cycles (for example with Vaporised Hydrogen Peroxide) but able to sustain a cleanroom VHP decontamination if requested.
Isolators
Air leak tightness classification of these systems is carried out following ISO 10648-2 standard and the isolation system will automatically check the actual leak tightness.
Due to its leak-free and extremely hygienic design, the isolator can be cleaned with semiautomatic cycles (WIP) highly effective in removing traces of contaminating products. The isolator can be decontaminated using agents in vapour phase as with Vaporised Hydrogen Peroxide, implementing fully automatic cycles.
Internally the isolator must assure a Grade A GMP environment, due to its leak-tight structure, and the surrounding production room can be classified as Grade C. Complete vial/syringe filling lines for aseptic and/or toxic products are installed in several countries worldwide.
As stated in the FDA guidelines and the recently approved new EU GMP Annex 1, isolators are recommended as the best technology for aseptic products.
The advantages offered in terms of final product quality and in terms of cost saving are clearly demonstrated, especially when a supplier such as IMA LIFE can provide the complete filling line and related isolators as a “single source”, decreasing engineering and validation costs, providing a standardised automation system and centralised data collection.
Advantages:
- Highest product protection
- Possibility of downgrading the production area to Grade C
- Humidity and temperature inside the Isolator can be controlled adopting a dedicated HVAC
- Modular HVAC design based on customer URS
- Integrated automatic decontamination cycles with Vaporised Hydrogen Peroxide
- Possibility of recycling the air used inside, saving HVAC energy consumption
- Full operator protection, useful with highly toxic and biohazardous products
- Possibility of performing WIP cycles (Wash In Place)
- Enhanced automation, full isolator-machine integration
- Opex saving (due to the downgrade of the surrounding production area from Grade B to C)
Weaknesses:
- Higher impact on building layout and utilities requirements
- Higher capex of the production line
- Higher complexity in commissioning and validation
Operation Cost Savings
Classification downgrade of the production room allows the following Operation Cost savings:
- Less quantity of air required by the production room
- Less time spent by operators entering/exiting the classified room
- Less expensive gowning
This is a typical filling line composed of:
- Accumulation table
- Filling and stoppering
- 2 Freeze Dryer loading systems
- Capping
The filling line installed with Open or Closed RABS will require unidirectional airflow inside the RABS + 50cm of open unidirectional flow at the operator side. With this configuration we need 20.6 m2 of Grade A (with unidirectional air flow) + 20.4 m2 of external unidirectional airflow (total 41m2) and 27 m2 of Grade B.
The same filling line installed with an Isolator will require unidirectional airflow inside the Isolator ONLY.
With this configuration we need 20.6 m2 of Grade A (with unidirectional airflow) and 47.2 m2 of Grade C.
Comparing different installations of the same filling line, the results are:
- The adoption of an Open or Closed RABS will allow reduction of the AIR Handling costs of approx. 35%, compared to installation in a conventional clean room.
- The adoption of an Isolator will allow reduction of the AIR Handling costs of approx. 64%, compared to installation in a conventional clean room.
- The adoption of an Isolator will allow reduction of the AIR Handling costs of approx. 45%, compared to installation with Open or Closed RABS.
These calculations do not take into consideration the possibility of air re-circulation. This possibility is available for all three configurations, but with the higher efficiency of Isolator technology.
Taking the strain out of sterile pharmaceutical and biotech production
Today’s pharmaceutical and biotech production sees technicians manually lifting, carrying and emptying sizable receptacles of sterile materials. Fragile components shall be handled with care, sterility assurance and containment is imperative, and we have to ensure that leaktight transfer is maximized at all times.
But manual material handling (MMH), if not supported by stringent SOP (Standard Operating Procedure) execution, may be prone to human error. There is also a growing occupational health issue, with the repeated strain of limbs, shoulders and backs making technicians susceptible to musculoskeletal injuries and chronic disorders that cause pain and even disability.
Optimizing the journey to the production line
For more than 50 years, the DPTE® system from Getinge has helped project leaders and process engineers achieve high-speed filling line production without compromising the integrity of aseptic transfer. The benefits of this system have now been extended through the use of an innovative, purpose-built mobile transfer platform – the DPTE® Transfer Trolley – to alleviate the reliance upon manual carrying and loading, and the associated risks and constraints.
The development of the DPTE® Transfer Trolley has followed extensive engagement with customer challenges and a close collaboration with IMA Life during the design review process. “This has enabled a new ergonomic design approach that addresses the needs of the operator while maintaining system integrity,” commented Sergio Manera, R&D Department, IMA Life.
Shortcomings of incompatible transfer platforms
The need for a purpose-built solution has become explicitly clear. We found many instances of tables and rigs being used to support technicians; helping transport large containers to the docking port and then elevate and hold them during the loading process. Analysis carried out in coordination with the IMA Life team showed some profound limitations with these devices. For example:
- Different loading ports within the same production environment may vary in height, whereas the device’s level is typically fixed;
- Materials require different elevations in order to evacuate at a safe speed (e.g. liquids, bulk powders and solid components), but single devices cannot meet these requirements without additional manual tilting;
- Most devices assume the use of the same container i.e. unable to suit all diameters and volumes and incompatible with both rigid, reusable canisters and single-use Polyethylene (or similar) bags;
- The manual task of stabilizing devices is often difficult and time-consuming, and can risk significant damage to isolator doors and walls;
- Large and/or heavy platforms occupy too much space and maneuvering them is likely to cause as many MMH-related injury risks as they prevent.
Working with partners like IMA Life, as well as numerous customers, Getinge has developed the DPTE® Transfer Trolley, a smart transfer platform for use with all DPTE® Beta solutions.
DPTE® Transfer Trolley – prioritizing safety for people, processes and products
The DPTE® Transfer Trolley is electrically assisted with enhanced ergonomics for ease of handling. Using it is almost effortless and, because it’s an integral part of the total DPTE® solution, sterility or containment are never compromised. In fact, the flexibility of the DPTE® Transfer Trolley extends the value of all DPTE® Alpha port environments through its cross-compatibility with DPTE-BetaBag® and DPTE® Beta Container solutions.
It has a broad elevation range up to 1800mm and can carry, rotate and incline (up to 90o) loads of up to 50kg to facilitate optimum aseptic transfer via any DPTE® system. Its native compatibility with DPTE-BetaBag® and DPTE® Beta Containers enables two format solutions from the same platform. All available diameters and volumes of each format can be supported with minor reconfiguration, the sole exception being the 460mm DPTE® Beta Container.
The DPTE® Transfer Trolley has a number of smart controls governing its safe and efficient handling. Minimal training is required to take advantage of its full maneuverability including all-wheel braking, precision motors for fine-tuned positioning, and multi-directional castors for maximum mobility.
In the opinion of Sergio Manera and his R&D colleagues at IMA Life in Ozzano dell’Emilia, Italy, perhaps its most ‘game-changing’ function is the ability to set and restore pinpoint alignments via its programmable memory. “We found this enables precision centering at speed, supporting safe and efficient docking of DPTE-BetaBag® and DPTE® Beta Container formats to the DPTE® Alpha port.”
All of these features are delivered from a compact, space-saving 1000mm x 700mm footprint that makes the DPTE® Transfer Trolley suitable for smooth operation within any cleanroom environment. Its pharma-grade finish and long-life rechargeable lithium batteries are also compliant with all relevant safety standards.
Time for a smart solution
The DPTE® Transfer Trolley extends the proven DPTE® system for aseptic transfer and addresses a missing link in the production process. As pharmaceutical and biotech companies continuously face increasing demands on production, which inevitably drive the need for more units to be transferred at greater frequency, production managers can harness the DPTE® Transfer Trolley to mitigate the risks of manual material handling, safeguard their employees, and focus on a safer, more efficient future.
