
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Convert the pre-formulated Ibuprofen DC 85 into a ready-to-use product.
Evaluating the performance of external lubrication to convert the pre-formulated Ibuprofen DC 85 into a ready-to-use tableting product.
Introduction
Recently, Continuous Manufacturing is a heavily investigated and discussed trend in the pharmaceutical industry. Key in this approach: the continuous supply of a tableting blend to a fast running, pilot or production scale rotary tablet press. This either requires the incessant incorporation of small quantities of a lubricant into a powder blend or the employment of external lubrication. The latter approach provides various advantages, e.g. the minimization of lubricant content in the tablet, which offers an inherent risk for incompatibilities. [1] In this investigation, directly compressible Ibuprofen (Ibuprofen DC 85 W) was used as model formulation. The tableting characteristics of this product have been well described [2, 3] allowing to focus on the efficiency of lubrication. The aim of this study was the evaluation of the performance of external lubrication in comparison to internal lubrication, as well as the scaling up from a lab scale to a production scale rotary press. Focusing on ejection force and tablet strength, three lubricants (magnesium stearate, stearic acid, and sodium stearyl fumarate) were tested in this study.
Materials and methods
Ibuprofen DC 85 W (BASF) is a pre-formulated (granulated), directly compressible material (Figure 1).

Figure 1: SEM image of Ibuprofen DC 85 W (SE detector, 5 kV, 12 nm Pt)
It contains 85% Ibuprofen, a binder (microcrystalline cellulose), a disintegrant (croscarmellose sodium) and merely requires the customised incorporation of some standard lubricant (e.g. magnesium stearate) to be ready for tableting. Three lubricants were evaluated in this study: magnesium stearate (Baerlocher), stearic acid (Kolliwax® S Fine, BASF), and sodium stearyl fumarate (Pruv®, JRS). Due to differences in lubrication efficiency, the lubricants were added in different concentrations, requiring slightly different tablet masses to deliver the required Ibuprofen dose (Table 1).
| Lubricant |
Content |
Content Ibuprofen DC 85 (%) |
Tablet mass (mg) | |
|
|
200 mg | 400 mg | ||
| Magnesium stearate | 0.5 | 99.5 | 236.7 | 473.4 |
| Stearic acid | 4.0 | 96.0 | 244.7 | 498.4 |
| Sodium stearyl fumarate | 3.5 | 96.5 | 243.3 | 487.0 |
| Ext. lubrication | tbd. | -100.0 | 235.3 | 470.6 |
Table 1: tablet formulations and resulting tablet mass (providing either 200 mg or 400 mg of Ibuprofen).
The lubricant concentrations (internal lubrication) were defined in a laboratory scale pre-test to achieve similar ejection forces for all formulations. The trials were conducted using Euro-D punches. To provide a dose of 200 mg Ibuprofen round-shaped, bi-convex punches (diameter 9 mm) were used. For compressing the higher dose (400 mg), oblong-shaped, bi-convex punches of the size 17×7 mm were chosen.
The first set of experiments were conducted on a lab scale rotary press (Prexima 80, IMA, Italy), equipped with a Euro-B/Euro-D turret holing four stations for each format. Generally, this equipment configuration is highly suitable for product development due to its flexibility regarding the use of different punch designs.
The second set of experiments (up-scaling) were conducted in a production scale rotary press (Prexima 300, IMA, Italy), equipped with an Euro-D turret (27 stations).
All the experiments (both scales) were performed applying a pre-compression force of 2.0 kN, and four different main-compression forces (about 6, 8, 10, 12 kN). During the up-scaling the parameters of feeder paddle speed, circumferential speed of turret and die filling time respectively, were kept constant.
On both machines the same external lubrication unit (LUMS, IMA, Italy) was employed. Parameters were selected accordingly to reduce the lubricant quantity provided to a minimum. Two different screw types were used to address the different volumes/flow characteristics of the lubricants.
Results and discussion
The general processability of the formulations were excellent, allowing for a constant tablet weight of low mass variability (SD <1%) independent of tableting scale and lubrication approach (internal [int], or external [ext]). Firstly, all formulations containing an internal lubricant were compressed as reference. Secondly, the parameters for the external lubrication system (LUMS) were determined for both punch geometries. It was found that magnesium stearate and sodium stearyl fumarate could be processed with very similar settings. The different powder flow characteristics of stearic acid required some adjustments, though. Both mixer speed and screw type (quantity sprayed) were changed to allow a proper application of the lubricant (Table 2, Table 3).
| 9 mm | 17×7 mm | ||||||
| m.u. | Magnesium stearate |
Stearic acid |
Sodyum stearyl fumarate |
Magnesium stearate |
Stearic acid |
Sodium |
|
| Air pressure | bar | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Mixer speed | rpm | 20 | 10 | 20 | 20 | 10 | 20 |
| Screw type | mm | 10 | 12 | 10 | 10 | 12 | 10 |
| Quantity sprayed | g/h | 418 | 750 | 373 | 347 | 1,572 | 373 |
| Screw speed | rpm | 100 | 45 | 100 | 80 | 108 | 100 |
Table 2: settings of LUMS at Prexima 80 for both punch geometries.
| 9 mm | 17×7 mm | ||||||
| m.u. | Magnesium stearate |
Stearic acid |
Sodyum stearyl fumarate |
Magnesium stearate |
Stearic acid |
Sodium |
|
| Air pressure | bar | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Mixer speed | rpm | 20 | 10 | 20 | 20 | 10 | 20 |
| Screw type | mm | 10 | 12 | 10 | 10 | 12 | 10 |
| Quantity sprayed | g/h | 347 | 750 | 341 | 420 | 1,080 | 380 |
| Screw speed | rpm | 80 | 45 | 80 | 108 | 80 | 108 |
Table 3: setting of LUMS at Prexima 300 for both punch geometries.
Atomic absorbance spectroscopy (AAS) allowed the subsequently analysis of the magnesium stearate and sodium stearyl fumarate content in the tablets.
The theoretical contents for the formulations carrying internal lubrication were confirmed. A magnesium stearate content of 0.05 to 0.15% could be detected for tablets where external lubrication was applied. The content of sodium stearyl fumarate was below the detection limit and couldn’t be determined. The ejection force measured during tableting, is the indicator for describing the efficiency of lubrication. Generally, it could be observed that external lubrication led to slightly higher but acceptable ejection forces compared to internal lubrication. However, the major driver was the type of lubricant used and not the way how it got introduced to the process. Independent of the lubrication approach, an increase in compression force had hardly any impact on the resulting ejection force value. This result and conclusion were applicable for both scales (Graph 1, Graph 2). Tablet strength that represents a quality measure, is generally affected by the lubrication approach. External lubrication fostered higher tablet strength, at least at higher compression forces.
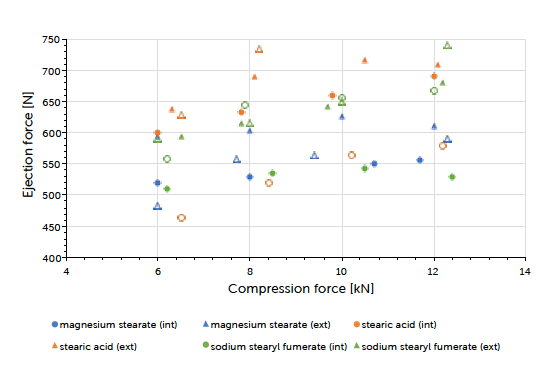
Graph 1: ejection force (mean value) vs compression force (mean value) and lubricant approach for Prexima 80 at turret speed of 50 rpm.
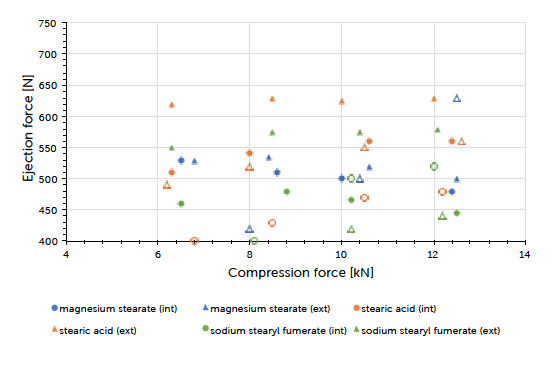
Graph 2: ejection force (mean value) vs compression force (mean value) and lubrication approach for Prexima 300 at a turret speed of 37 rpm (9 mm punches filled symbols, 17×7 mm punches unfilled symbols).
Equal results were seen in both scales (Graph 3, Graph 4). Increasing the tableting speed at Prexima 300 from 37 rpm up to 80 rpm hardly changed either tablet strength nor ejection force. This proves the robust processability of Ibuprofen DC 85 W that allows fast processing/high output without requiring any adjustment of equipment or process parameters.
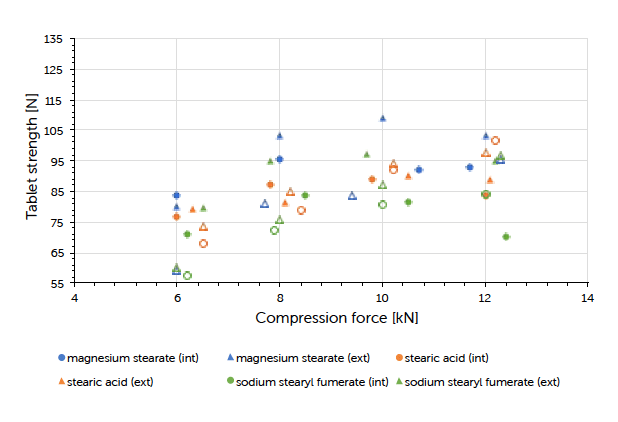
Graph 3: tablet strength (mean value, n=20) vs compression force (mean value) and lubrication approach for Prexima 80 at a turret speed of 50 rpm (9 mm punches filled symbols, 17×7 mm punches unfilled symbols).
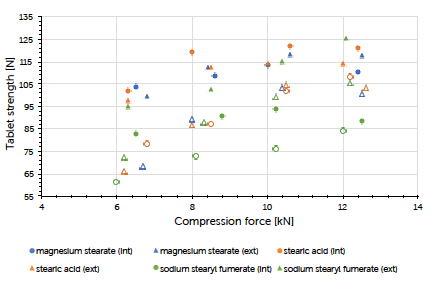
Graph 4: tablet strength (mean value, n=20) vs compression force (mean value) and lubrication approach for Prexima 300 at a turret speed of 37 rpm (9 mm punches filled symbols, 17×7 mm punches unfilled symbols).
Conclusion
The introduction of external lubrication successfully converts the directly compressible Ibuprofen DC 85 W into a ready-to-use tableting product, allowing the continuous production of tablets without requiring an up-stream blending step.
External lubrication offers the possibility to reduce lubricant quantity required in the tableting formulation, improving tablet strength at the same time.
Furthermore, it has no impact on the performance of the tablet press, allowing efficient manufacturing of Ibuprofen tablets.
LUMS proves to be a very flexible auxiliary equipment that could easily process different kind of lubricants. Additionally, implementation at different scale rotary presses (pilot and production scale) is straightforward and hardly requires any adjustments.
References
[1] Bharate S. S., Bharate S. B., Bajaj A.N., Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review, “J. Excipients and Food Chem.”, 1 (3), 2010.
[2] Bang F., Cech T., Schlindwein W., Evaluating critical quality attributes of direct compressible ibuprofen in a QbD approach, 10th PBP World Meeting, Glasgow, United Kingdom, April 4-7, 2016.
[3] Bang F., Cech T., Otterbach S., Rottmann N., Evaluating critical quality attributes of direct compressible Ibuprofen in a QbD approach on a rotary press, 11th PBP World Meeting, Granada, Spain, March 19-22, 2018.


