
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |The potential of Croma continuous coater.
Jola Gorreja, R&D Process Engineer at IMA Active Guia Bertuzzi, Product Manager for process equipment at IMA Active |
1. Introduction
The modern pharmaceutical industry is facing the challenge of transition from a traditional manufacturing approach based on batch-wise production to Continuous Manufacturing.
Croma continuous coater brings an innovative technological progress in manufacturing based on modularity and truly continuous film-coating of tablets. Croma enables faster cycle times with great process flexibility, optimized yield and throughput by enhancing sustainability.
Comparing with the traditional batch coating technology Croma provides an extremely efficient process, which allows also easy scale-up. Coating process becomes more efficient: coating time is reduced, as well as the exposure of the tablets to the coating environment, with a significant impact on reducing manufacturing cost.

2. Purpose
Coating uniformity and tablet appearance are evaluated using Croma coater either by batch or continuous modes. Trials are carried out to evaluate he effect of tablets residence time versus coating uniformity.
The increase of tablets throughput and the consequent decrease of tablets residence time do not affect the final surface appearance of the coated tablets.
3. Croma technology
Croma continuous coater’s working principle is based on a traditional coating system: tablets mixing in a continuous flow through a rotating perforated drum invested by hot air flow, in the meantime they are coated on the way through spray guns. Croma has been dimensioned on typical tablets throughput of a tablet press so it is possible to integrate it also downstream it, enabling a continuous production of coated tablets. However required throughput and coating weight gain are achieved by combining Croma modules.
4. Truly continuous mode
The breakthrough of Croma stands in consistently regular tablets flow versus coating suspension flow.
Drum and mixing baffles provide a continuous motion of tablets, thus keeping them free flowing under control with no risk of back-flow. Unlike in batch coating, in continuous coating it is important to ensure not only a good radial mixing but also a good axial transportation of tablets along the drum. The feeding of the tablets is ensured by means of a loss in weight feeder and the discharging by means of a patented transfer system. Axial transportation depends on the feeding flowrate of tablets and from the discharging point. The drum speed varies to handle different tablets shape and dimensions.
A constant throughput is achieved thanks to the monitoring of the tablets bed level. A proper tablets bed ensures high frequency of their presence under the spray area and low residence time in the drum. A full covering of the surface bed during spraying is also a key parameter to reach a good intra and inter tablet coating distribution. While drying of tablets takes advantage of the high tablet-to-heat transfer air exposure associated with the rolling/cascading mode.
5. Batch mode
Croma can work also in batch mode with a batch size of 7-9 kg depending on the type of tablets. In this case the tablets are still by the feeder, the discharge port is kept closed and once the tablets bed is displayed all along the drum the coating process can start and is managed as in a conventional coating pan, spraying the coating suspension until the desired weight gain. The batch process is very fast, about 5- 15 min depending on the quantity of coating suspension and spraying rate. Batch mode can also be considered as a clever tool to find the right process parameters for continuous mode for any type of tablet and coating suspension to use. Concerning continuous coating process, each Croma module has a fixed average range of tablet throughput (30-80 kg/h) depending on many factors:
- Weight gain achievable
- Spray flowrate achievable
- % of polymer in suspension
- Tablet shape
Each of these factors influence the mean throughput of the machine.
Once target weight gain and the percentage of solid in the coating suspension are known, it is possible to calculate the tablets throughput, since
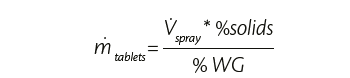
Tablets throughput depends on the quantity of coating suspension to spray which in turn depends on the heating capacity of the system, on the polymer nature and the physical and chemical properties of the tablets. Energy and mass balances are also considered to find the starting process parameters like process airflow rate and temperature. Later they are adjusted to consider potential loss, tablets temperature (if required) and final tablets appearance.
6. Materials and methods
- Uncoated tablets: placebo oblong tablets and round biconvex. The composition of the tablets and their properties are shown in Table 1.
- Coating suspension: Opadry II Blue 85F205098 (Colorcon Ltd, Dradford, UK). The film coating suspension is prepared at 20% of solids concentration (w/w) in water and applied to a target weight gain (WG) of 3% (w/w).
| Shape | Dimensions (mm) |
Excipients | Average Hardness (N) |
Bulk density (kg/m3) |
Average |
SD (mg) |
| Oblong | 17 x 7 | Lactose 88% Mg-Stearate 5% AL2O3 5% Cellulose in powder: 2% |
140 | 770 | 600 | 2.16 |
| Round biconvex | 9 | Lactose 88% Mg-Stearate 5% AL2O3 5% Cellulose in powder: 2% |
90 | 810 | 265 | 1.99 |
Table 1: placebo tablets characteristics.
7. Experimentals
Batch and continuous trials are carried out, for each tablet shape, in a single Croma module.
The coating pan is fed by a loss in weight feeder until the right hold- up is reached. Then coating is carried out in batch mode.
When working in continuous mode, tablets throughput is varied from lower to higher rates, 40 to 81 kg/h still by regulating the feeder upstream Croma. Increasing the throughput the tablets mean residence time inside the drum decreases.
The variation of tablets throughput determines the variation of spray rates and the other process parameters, as shown in Table 2. The inlet air temperature is adjusted to maintain the product temperature constant.
| Process Parameters | |||||||
| Tablet shape /dimension |
Croma |
Spray rate (g/min) | Inlet Air flowrate (m3/h) |
Inlet |
Outlet |
Residence |
Pan |
| Round 9 mm | Batch | 150 | 750 | 75 | 46.5 | – | 6.7 |
| 40 | 100 | 750 | 72 | 47.5 | 10 | ||
| 48 | 120 | 750 | 74 | 48.5 | 8.4 | ||
| 60 | 150 | 750 | 75 | 46.5 | 6.7 | ||
| 72 | 180 | 950 | 78 | 45.8 | 5.6 | ||
| 81 | 202.5 | 1,050 | 78 | 45.6 | 5 | ||
| Oblong 17 x 7 mm | Batch | 150 | 800 | 72 | 45.5 | – | 7.3 |
| 40 | 100 | 700 | 70 | 46 | 11 | ||
| 54 | 135 | 850 | 70 | 45 | 8.1 | ||
| 60 | 150 | 800 | 72 | 45.3 | 7.3 | ||
| 72 | 180 | 850 | 77 | 46.8 | 6.1 | ||
| 81 | 202.5 | 1,000 | 79 | 46.5 | 5.4 | ||
Table 2: process variables.
8. Sampling
In batch mode, samples are taken in 3 different positions (front, middle and back of the coating drum) at 2% and 3% of weight gain. In continuous mode samples are taken at the exit of the drum at various intervals of time for each throughput.
9. Coated tablets evaluation
Color uniformity of 20 tablets for each sample is analyzed using a portable color spectrophotometer (ColorEye XTH, X-Rite Corporated). The data is analyzed using the (CIE) L* a* b* System. In CIELAB color space, the colors are described by three numerical values: the lightness intensity (L) and 2 chromatic components (a, b).
The color difference of the sample from a standard is called ΔE.
∆E = [(L1–L2 )2 + (a1–a2 )2 + (b1–b2 )2]1/2
In general deltaE < of 3 is considered acceptable.
10. Results and discussion
For the 17×7 mm tablets, batch coating is performed by spraying 150 g/min on a hold-up of 7.3 kg of tablets. After 4.9 min (2% w.g.) and 7.3 min (3% w.g.) samples are taken in the three positions of the drum described above. For each sample, color uniformity is measured as it can be shown in Figure 1.
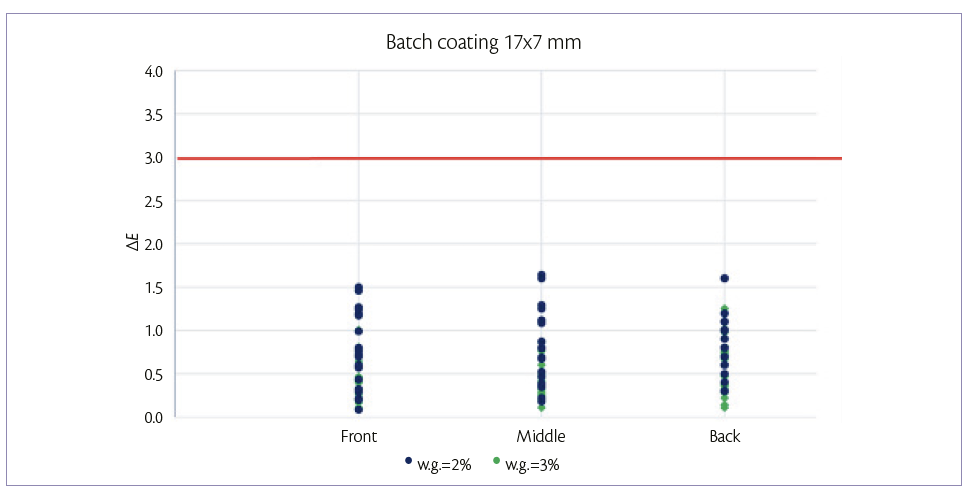
Figure 1: color uniformity of 17×7 mm tablets at 2% and 3% of W.G. batch coating mode.
For the 9 mm tablets, batch coating is performed by spraying 150 g/min on a hold-up of 6.7 kg of tablets. After 4.5 min (2% w.g.) and 6.7 min (3% w.g.), samples are taken in the three positions of the drum described above. For each sample, color uniformity is measured as it can be shown in Figure 2.
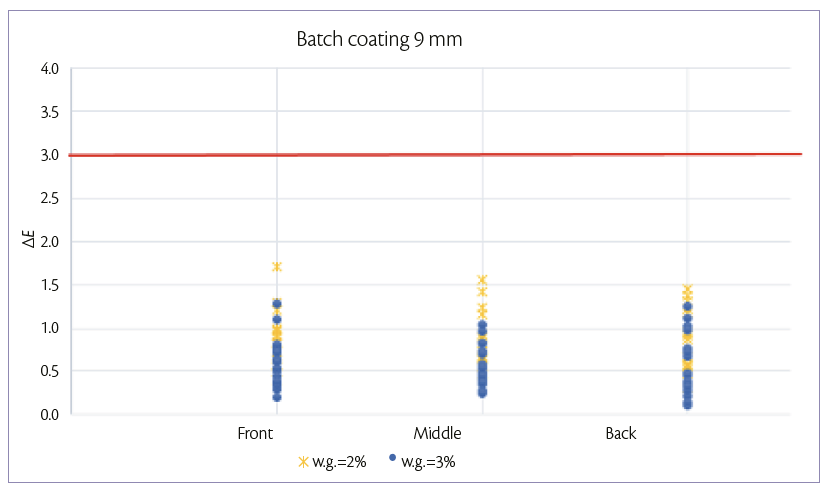
Figure 2: color uniformity of 9 mm tablets at 2% and 3% of W.G. batch coating mode.
Figures 1 and 2, show that, for both tablets shapes, it is possible to achieve a good tablet color distribution (ΔE <3) in all the length of tablet bed and at low weight gains also.
During continuous coating mode, the weight gain achievable is maintained constant and equal to 3%. When tablets feeding is increased also the other parameters are adjusted according to the increase of the spray rate. For each tablet shape, the 5 processes are performed in the same day, changing the throughput every 1 hour. Samples are taken at the discharge point of the pan coater for each tablet flowrate.
The ΔE variation at different tablet flow rates can be shown in Figures 3 and 4.
In both processes , the color difference between samples and target is <3.
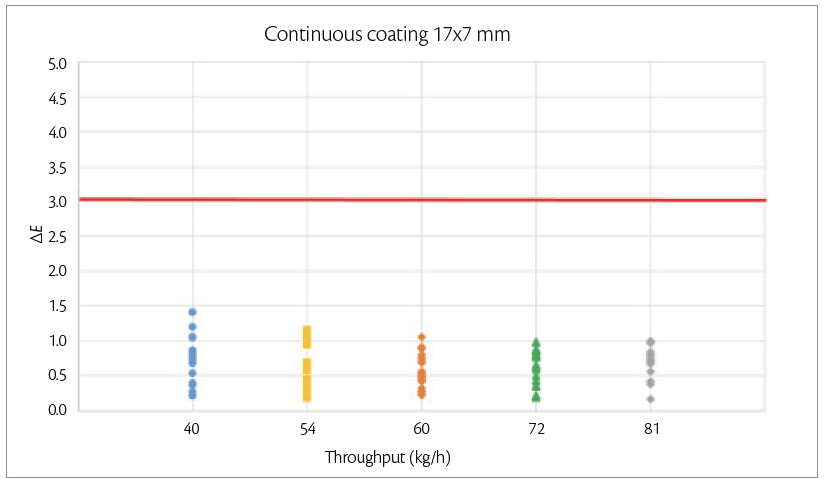
Figure 3: color uniformity of 17×7 mm tablets at 3% of W.G. continuous coating mode.
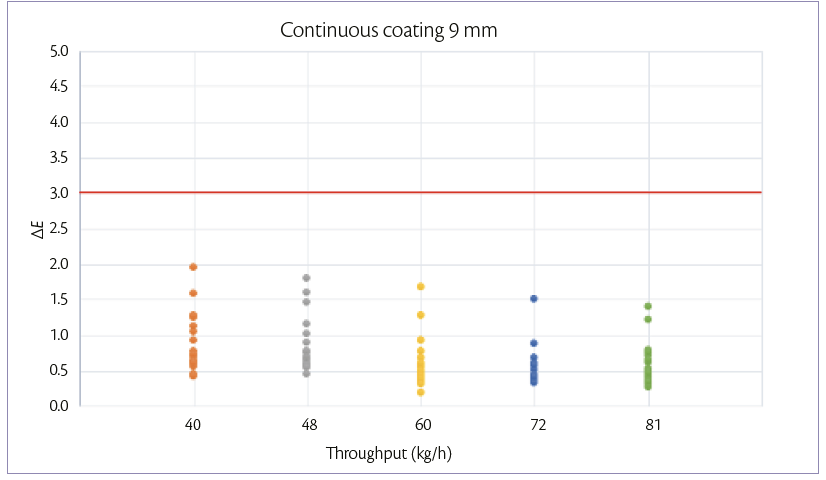
Figure 4: color unifomity of 9 mm tablets at 3% of W.G. continuous coating mode.
11. Conclusions
In this study, it is shown that Croma single module configuration provides the ability to continuously coat the tablets at different flow rates. In addition, it is suitable to succeed also batch coating with same excellent results. In fact homogeneous quality is achieved both in continuous and in batch mode operation.
References
Kevra A., Evaluation of a Film Coating that Produces Enhanced Tablet-to-Tablet Film Uniformity, Reprint of poster presented at “AAPS” , November 2005.
Advancement of Emerging Technology Applications to Modernize the Pharmaceutical.
Manufacturing Base Guidance for Industry, Office of Pharmaceutical Quality and the Office of Compliance in the Center for Drug Evaluation and Research at the Food and Drug Administration, December 2015.
Freireich B., Wassgren C., Intra-particle coating variability: Analysis and Monte-Carlo simulations, “Chemical Engineering Science”, 65 (2010), 1117–1124. Boehling P., Toschkoff G., Knop K., Kleinebudde P., Just S., Funke A., Rehbaum H., KhinastChen W., Chang S.-Y., Kiang S., Marchut A., Lyngberg O., Wang J., Rao V., Desai D., Stamato.
J.G., Analysis of large-scale tablet coating: modeling, simulation and experiments, “Eur. J. Pharm. Sci.”, 90, 14–24, 2016 b.
Chen W., Chang S.-Y., Kiang S., Marchut A., Lyngberg O., Wang J., Rao V., Desai D., Stamato, H., Early W., Modeling of pan coating processes: prediction of tablet content uniformity and determination of critical process parameters, “J. Pharm. Sci.”, 99, 3213–3225, 2010.
Suzzi D., Radl S., Khinast J.G., Local analysis of the tablet coating process: impact of operation conditions on film quality, “Chem. Eng. Sci.”, 65 (21), 5699–5715, November 2010.
Suzzi D., Toschkoff G., Radl S., Machold D., Fraser S.D., Glasser B.J., Khinast J.G., DEM simulation of continuous tablet coating: effects of tablet shape and fill level on inter-tablet coating variability, “Chem. Eng. Sci.”, 69 (1), 107–121, 2012.
http://zschuessler.github.io/DeltaE/learn/
Paper Sections:
Last Submitted Papers:
- How to step-up metformin tablets production from a pilot scale coater to three different industrial scale equipment.
- How to enhance tableting production with a paracetamol based formulation.
- Metformin manufacturing: scale-up from middle-size to large-size rotary tablet press.
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
