
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |An aid-instrument for data analysis and interpretation in tableting: a case study of elastic and plastic formulations.
|
|
1. Introduction
Galen was an ancient Greek physician, an expert also in anatomy, physiology, pathology, logic and philosophy, from whom the association with galenic pharmacy and pharmaceutics probably derives: his broad-ranging talents gave him the attribution of the first formulation scientist.
Pharmaceutics is the study of how to optimise the administration of a medicine, through a rational scientific design. It is a multidisciplinary science that involves a wide spectrum of fields with the aim of developing a formulation for a certain route of drug delivery: topical, nasal, parenteral, ocular, pulmonary, rectal, vaginal and oral. [1]
The focus of this article is the oral route, in particular the tablet, which is clearly the best known and most used. Several advantages can be obtained by manufacturing tablets: dosage accuracy, ease of administration, high stability and patient compliance. The pharmaceutical industry has to produce tablets that are characterised by high quality: uniform mass, strong enough to be handled, packed and stored but also capable of disintegrating and releasing the API in a desired manner through alimentary canal. [2]
Deep knowledge of raw materials and manufacturing processes is essential to achieve this target. In the development phase of a tablet formulation, several aspects should be taken into consideration: material features on one hand and machine characteristics on the other. Ideally, the particles of a final mixture to be tableted should be free flowing, uniform within a tight range of particle size distribution, cohesive enough to lock and hold together but not so much as to adhere to metal surfaces. All these needs must be met while at the same time considering the inner proprieties of each compound within the formulation: API, filler, disintegrant, binder, colorant and lubricant, whose combination generates a specific and non-predictable behaviour.
A large number of investigations have been carried out over the decades and several theories of tablet bonding mechanisms have been discovered. However, there is still no one universal theory that could be used to predict the proprieties of compressed tablets: from this concept, IMA Active Division has developed IMAGO, whose name originates from the Latin imago, imaginis. It is an analytical software that helps scientific people interpret data from tablet press and tablets analysis; it is an instrument that acquires and processes information to understand the whole tableting process.
The aim of this article was the study, through IMAGO, of two different formulations with an already-known behaviour (plastic and elastic) by changing the tableting process parameters on a small-scale rotary tablet press, model Prexima 80.

2. Materials and methods
Two different blends were used as references for the study: the first with a prevalently plastic behaviour, lactose based (Table 1), the second more elastic, starch based, as reported (Table 2).
| Components | Percentage (%) |
| Tablettose 80 | 66.33 |
| Vivapur 102 | 33.17 |
| Magnesium Stearate | 0.50 |
Table 1: Plastic formulation.
| Components | Percentage (%) |
| Starch 1500 | 89.55 |
| Vivapur 102 | 9.95 |
| Aerosil 200 Pharma | 0.25 |
| Magnesium Stearate | 0.25 |
Table 2: Elastic formulation.
Both blends have been obtained through mixing in a tumbler blender, model Cyclops Mini, then tableted in standard conditions (standard die feeder with flat paddles installed) on a small-scale rotary tablet press, model Prexima 80 (IMA, Italy). This machine is the most suitable for R&D purposes of Prexima series: indeed, it was equipped with a mix-turret that can host both Euro-D than Euro-B punches. For this specific study, Euro-B 9 mm biconvex punches have been used.
The behaviour of both blends has been studied using a non-full factorial D.o.E. approach for a total of 48 trials. The variables studied were 4 levels of main compression force (5, 10, 15 and 20 kN), 3 levels of pre-compression force (0, 1/3 and 1/2 of main compression force), turret speed (30 and 50 rpm) and upper punch penetration (2-2.5 mm and 2-4 mm) respectively on precompression and final compression. Thanks to IMAGO application, the curves forces-time as well as tabletability, compactability and compressibility have been the focus of the study, to better understand powder behaviour under tableting.
3. Results
Both blends have been tableted without any particular issue: smooth processes have been obtained with no mechanical or process adjustment. Weight has been kept constant (223 mg on average) in every trial to obtain comparable results.
The trend of the thickness and the tablet strength (hardness) are independently similar to process the parameters and variables studied: for simplicity, only a demonstrative condition, at different compression forces (CF) is reported (Figure 1 and 2) to summarise the whole behaviour within the D.o.E.
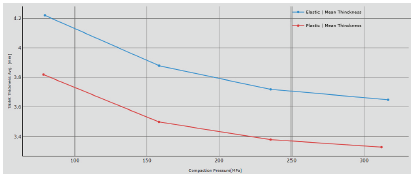
Figure 1: Thickness variation at different CF.
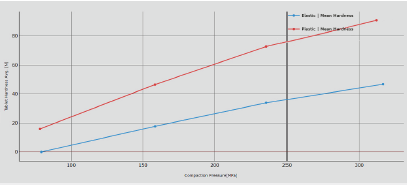
Figure 2: Hardness variation at different CF.
The red line is the plastic formulation whereas the blue is the elastic one: on both it is possible to note that if the thickness decreases, the hardness increases; however the plastic formulation allows for thinner tablets with enhanced tablet strength. The main explanation is due to the powder’s intrinsic characteristics: the plastic formulation tends to form solid and stronger bonds. The elastic particles relax and try to recover their original position after pressure is applied: the result is higher thickness than the plastic formulation, in the same processing conditions, and lower tablet strength value. It is also interesting to note that using a pressure of 50 MPa for the elastic blend is not sufficient to get a compact tablet, whereas for the plastic powder the average tablet strength is around 17 N.
The forces profile visualisation instantly shows the difference between the two formulations (Figure 3): the violet curves, representing the plastic blend, have narrower peaks compared to the elastic formulation. Furthermore, it is evident how plastic powder offers higher consistency and reproducibility than the elastic formulation, which shows a wider variation among the various recorded profiles. As before, the main reason is the intrinsic tendency of the material: the plastic particles tend to be more compact under an applied pressure.
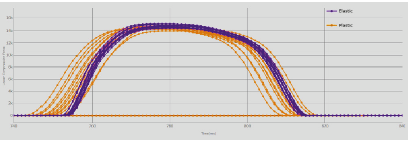
Figure 3: Compression forces trend comparison between plastic (violet) and elastic (orange) formulation.
The use of pre-compression and lower tablet press speed means the differences between peak shape can be reduced: the values of the main compression chosen do not affect the overall profile of the trends. Tabletability (Figure 4), compressibility (Figure 5) and compactability (Figure 6) can finally better define powder behaviour.

Figure 4: Tabletability trend.
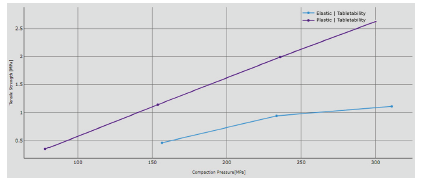
Figure 5: Compressibility trend.
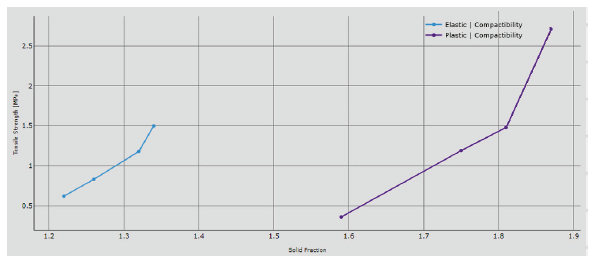
Figure 6: Compactability trend.
Plastic formulation can reach a higher value of tensile strength at the same pressure applied, providing an enhanced tendency to be transformed into tablet. The compressibility confirms that solid fraction is higher for the plastic blend: 1.81 can be reached compared to the 1.28 obtained for the elastic formulation. It is well known that particles with elastic behaviour try to recover their original position against the reduction of the volume induced by the applied force.
The compactability graph definitively confirms the hypothesis: the lower tensile strength and solid fraction obtained on the elastic formulation gives the left-sided curve, while the plastic one is on the right; the evidence is clear that the elastic formulation tends to remain more compact under an applied pressure. The evidence is that use of pre-compression improves all values of tensile strength or solid fraction, particularly at the higher chosen tablet press speed: the reduced dwell time worsens the tablet strength and increases the porosity of the tablet even for the plastic formulation. The upper punch penetration strongly affects the compactability graph: working deeper inside the dies allow for a more symmetric compression and, consequently, increased the tensile strength.
4. Conclusions
IMAGO software is definitively the proper aid instrument for data analysis in interpreting powder behaviour: more specifically, elastic formulation needs higher pre-compression value and lower tablet press speed to achieve compact tablets.
The forces graphs predictably highlighted a narrower peak for the plastic formulation compared to the elastic blend: the intrinsic tendency of the powder can be summarised and analysed by studying these trends easily displayed by IMAGO.
Tabletability, compactability and compressibility always evidenced a lower value of tensile strength or solid fraction on elastic formulation compared to the plastic blend: the use of IMAGO allows useful information to be gained by comparing different tests performed within the D.o.E.
References
[1] Mahmoodi, F., Compression Mechanics of powders and Granular Material Probed by Force Distributions and Micromechanically Based Compaction Equation, “Dissertations from the Faculty of Pharmacy”, ActaUniversitatisUpsaliensis, Digital Comprehensive Summaries of Uppsala, Uppsala, 2012. [2] Antikainen, O.K., New Method to Evaluate Applicability of Powders and Granules for Tablet Compression, “DisserttionesBiocentriViikkiUniversitatisHelsingiensis”, 28/2003, p. 64, 2003.
Paper Sections:
Last Submitted Papers:
- Influence of material and capsule filling process with Minima on aerosolization performances by DPIs.
- The potential of Croma continuous coater.
- How to step-up metformin tablets production from a pilot scale coater to three different industrial scale equipment.
- How to enhance tableting production with a paracetamol based formulation.
- Metformin manufacturing: scale-up from middle-size to large-size rotary tablet press.
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
