In recent years, tablet press technology has continued to evolve quickly. Today’s tablet presses allow for detailed monitoring of the compression characteristics of each individual tablet.
Challenging the processing performance of the all-in-one tableting aid Kollitab™ DC 87 L in direct compression, using a high-speed, production-scale rotary press, and evaluating the impact of different tablet geometries on tableting speed.
Introduction
In recent years, tablet press technology has continued to evolve quickly. Modern equipment combines higher performance, improved mechanical features, smart control systems with a user-friendly Human-Machine Interface (HMI), and an appealing design.
State-of-the-art measuring systems represent an additional, major evolution. Today’s tablet presses allow for detailed monitoring of the compression characteristics of each individual tablet. This enables the implementation of quality control measures already during production, by rejecting individual tablets being produced under conditions that might possibly affect the quality attributes of the drug products. Consistently, modern rotary presses do not only improve performance in production, they additionally ensure product quality and reliability, by minimising the risk of batch failure.
All these innovations arise with an increased effort regarding validation requirements. Moreover, continued process verification (CPV) is required, to periodically review equipment performance and make sure that any kind of malfunction of probes or sensors is noticed in good time.
One should be aware that before modern tablet presses start their daily routine in a pharmaceutical production unit, the equipment necessarily requires various qualifications that must be fully accomplished during the factory acceptance test (FAT) or the site acceptance test (SAT):
- Installation qualification (IQ)
- Operational qualification (OQ)
- Process performance qualification (PPQ)
The acceptance tests are qualifications which are commonly not product-related. Focus is set on the performance of the equipment and its operation within the pre-defined specification limits. This test is performed twice, firstly at the equipment manufacturer’s site (FAT) and secondly at the final operational site (SAT).
The subsequent performance qualifications reflect the documented verification that the instrument can perform effectively and in a reproducible manner within a given specification. At the same time, it allows one to determine the processing range associated with potential manufacturing risks and faster investigation of deviations that may occur during the process. Particularly for tablet presses, it is essential to verify the ability of the machine to perform high-speed tablet production, while assuring the production of tablets is perfect in terms of quality target, as well as from an aesthetic point of view [1, 2].
All qualification procedures have one aspect in common: they focus on the performance of the equipment. To avoid unnecessary variations, a standardized product is required to limit the variations deriving from the tableting blend, to really focus on the reliability of the overall process including probes and sensors. This is where Kollitab™ DC 87 L comes in useful. Kollitab™ DC 87 L is an all-in-one tableting aid which contains all the necessary components to formulate an immediate-release tablet. In the case of placebo tablet production, the product converts into a drum-to-hopper product, meaning it can be processed without any additional blending steps. While continuously providing the same, its use in qualification procedures takes away complexity and potential sources of measuring inconsistencies. Being a pharma excipient, it can be processed at equipment manufacturers as well as in the GMP environment of a pharma production site.
The aim of this study is the evaluation of Kollitab™ DC 87 L for the purpose of equipment qualification in a robust and intensely tested rotary tablet press model Prexima 300 (IMA, Italy) [3]. Focus is put on the performance test of a high-speed rotary press and the quality attributes of the resulting tablets. In addition, different tablet geometries are tested to prove the general suitability of the product.
Materials and methods
Kollitab™ DC 87 L (BASF) is an “all-in-one” tableting aid. It provides all functionalities required to formulate an immediate release tablet. The co-processed excipient comprises the following components: 87% of lactose monohydrate as filler, Kollidon® CL-F (crospovidone) as disintegrant, Kollicoat® IR as binder, and sodium stearyl fumarate as lubricant [4].
The ready-to-use excipient was tested on a rotary tablet press Prexima 300 (IMA, Italy) equipped with 2 exchangeable turret types: Euro-D turret hosting 27 stations with two punches for shape and dimension, oblong biconvex Ø 17×7 mm and round biconvex Ø 25 mm; Euro-B turret hosting 41 stations and BB round biconvex punches Ø 9 mm (Figure 1).

Figure 1: Punch shapes used for the investigation.
Focus of the tests was to reach the highest output possible for each punch geometry and to evaluate the final tablet characteristics in a continuous production session of one hour. The following parameters were evaluated: process stability, average weight and average tablet strength with their relative standard deviations (RSD).
Results and discussion
Kollitab™ DC 87 L is a free-flowing, dust-free material, allowing for easy handling. The drum-to-hopper concept can easily be implemented by the operator without taking special precautions (e.g., dust exhaustion installations). As the Prexima 300 was equipped with an automatic feeder, the excellent flowability and formulation consistency enable high-quality tablet compression throughout the entire process.
Oblong punches, 17×7 mm
With the oblong punches 17×7 mm, tablets with an average weight of 580 mg, an average thickness of 5.4 mm, and an average tablet strength of 165 N were produced. RSD on critical features (weight [Figure 2] and tablet strength [Figure 3]) and stability of the process allowed the tablet press to run at the full nominal speed (120 rpm, 194,400 tab/h) with a pre-compression force (PCF) of 8 kN and a main compression force (CF) of 20 kN.
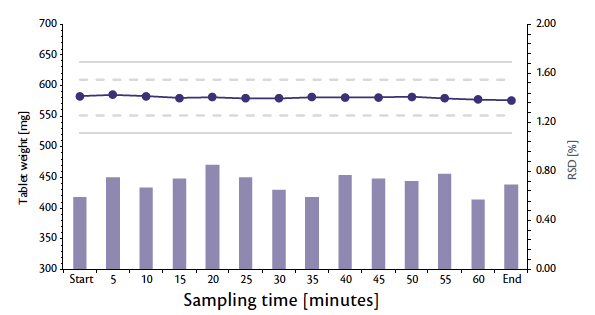
Figure 2: Weight trend and RSD during 1 hour of production at full nominal tablet press speed.
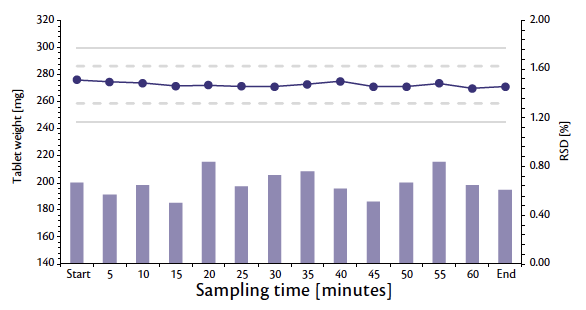
Figure 3: Tablet strength trend and RSD during 1 hour of production at full nominal tablet press speed.
Round-shaped punches, 25 mm
With a 25 mm punch shape, tablets of approx. 3,000 mg, a thickness of 6.6 mm, and a tablet strength of 140 N were obtained. Compared to the previous trial, as the tablet volume and mass respectively increased consistently, the performance had to be adjusted to 120,000 tab/h. The tableting speed required manual eeding of one commercial pack of Kollitab DC 87 L every 180 seconds into the tablet press hopper, proving the excellent flow- and processability of the excipient, maintaining considerable tablet press output with a tablet of 3,000 mg as average weight.
The tableting process went smoothly, applying 13 kN as PCF and 40 kN as CF. Worth noting, that given the high throughput, there was no change in tablet constant, further proving the excellent performance of both Kollitab™ DC 87 L the Prexima 300 in high-performance processes.

Figure 4: Weight trend and RSD during 1 hour of production at 120,000 tab/h.

Figure 5: Tablet strength trend and RSD during 1 hour of production at 120,000 tab/h.
Round-shaped punches, 9 mm
The study was concluded with a final test using 9 mm punches, mounted on a Euro-B turret, hosting 41 stations. The previous results were confirmed: at full nominal turret speed (194,400 tab/h), a PCF of 7.7 kN, and a CF of 18.5 kN, tablets were obtained with stable and constant characteristics (mass: 270 mg, diameter: 4 mm, and breaking force: 170 N). Both, weight (Figure 6) and tablet strength (Figure 7) trends demonstrate a reliable and robust process, similar to the previous results with the other punches.

Figure 6: Weight trend and RSD during 1 hour of production at full nominal tablet press speed.

Figure 7: Tablet strength trend and RSD during 1 hour of production at full nominal tablet press speed.
Conclusion
Kollitab™ DC 87 L proved to be a 16.00 excellent all-in-one tableting aid. The co-processed excipient shows excellent performance in a drum-to-hopper concept, in which it is used as a placebo to support machine qualifications. Even though the focus of Kollitab DC 87 L lies with the simplification of drug product development, it is also capable of making manufacturing easier, in the case of performance tests on tableting equipment. The processing reliability allows for focus on the qualification of the equipment, without the risk that variations are caused by the tableting blend.
This high degree of blend reliability, combined with the fine-tuning in process optimisation and robust structure of the Prexima 300 allows for continuous processing, being constant and reproducible throughout the entire manufacturing cycle of a tableting process. Different punch types were investigated at the maximum speed of the tablet press, obtaining tablets with a general weight RSD below 1%.
References
[1] Choudhary A., Performance Qualification (PQ) of Pharmaceutical Equipment, Pharma guideline
[2] Choudhary A., Importance of Validation in Pharmaceuticals, Pharma guideline
[3] https://ima.it/pharma/machine/prexima/
[4] https://pharma.basf.com/products/kollitab-dc-87-l
Relive
Achema
The exclusive tech videos shot during Achema 2024 are now available on our dedicated website


