
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Metformin manufacturing: scale-up from middle-size to large-size rotary tablet press.
Federica Giatti, Compression Technologist at IMA Active Fabriano Ferrini, Product Manager for tablet presses at IMA Active |
1. Introduction
Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin, a hormone that regulates blood sugar, or when the body cannot effectively use the insulin it produces. Hyperglycemia, or raised blood sugar, is a common effect of uncontrolled diabetes and over time leads to serious damage to many of the body’s systems, especially the nerves and blood vessels.
In 2014, 8.5% of adults aged 18 years and older had diabetes. In 2016, diabetes was the direct cause of 1.6 million deaths and in 2012 high blood glucose was the cause of another 2.2 million deaths.
Type 1 diabetes (previously known as insulin-dependent, juvenile or childhood-onset) is characterized by deficient insulin production and requires daily administration of insulin.
Type 2 diabetes (formerly called non-insulin-dependent, or adult-onset) results from the body’s ineffective use of insulin. Type 2 diabetes comprises the majority of people with diabetes around the world, and it is largely the result of excess body weight and physical inactivity [1].
Metformin is a first line agent for the treatment of type 2 diabetes, it helps to control the amount of glucose in blood, decreasing the amount absorbed from food and made by the liver, and increases body’s responses to insulin [2].
A majority of currently available metformin formulations are immediate-release (IR) products, which release the whole drug within 1-2 hours after dosing, resulting in high drug concentration in the gastrointestinal tract (potentially with undesired adverse effects). This drug formulation represents a regular version, taken at meal time three times a day at dosage of 500, 850 and 1000 mg. To counteract such common gastrointestinal side-effects and to enhance patients compliance, metformin slow-release (SR) and extended-release (XR) tablets were introduced, allowing once daily dosage. Such newer formulations release the active drug via hydrated polymers, which expand after the uptake of fluid.
Aim of the study is the process optimization, from dispensing of raw materials to coating of metformin hydrochloride formulation in order to maximize the output of the tablets produced maintaining high quality of all the pharmaceuticals forms.


2. Materials and methods
A formulation metformin based (IR) 25 kg batch size was dispensed, granulated, compressed and coated with IMA equipment. Four batches were manufactured (Met1, Met2, Met3, Met4) in automatic mode. After dispensing, powders were granulated with a binding solution in a pilot high shear mixer granulator, (Roto Mix 60, IMA) and subsequently dried in a pilot fluid bed (Aria 120, IMA). After dried, the milled granule were finally mixed in a tumbler blender (Cyclops, IMA) and then compressed in a rotary tablet press (Prexima 300, IMA). Final coated tablet were obtained in a pilot perforated coating machine (Perfima Lab, IMA). Tableting was then scaled-up in a large-industrial size rotary double side tablet press (Prexima 800, IMA) to enhance production output.
3. Manufacturing procedure
API micronized metformin was sieved through a 200 micron sieve. All the excipients (except the PVP–K30) were also pre-sieved and then dispensed accordingly to the quantity needed for 25 kg batch size.
The selected quantity of binder (PVP-K30) was dissolved in water in order to prepare the binding solution while the rest of quantity was added as dry binder.
Once granulated, the wet mass was unloaded from the HSMG and de-agglomerated and subsequently loaded in the fluid bed by vacuum to be dried until the target based on the product temperature and confirmed by the moisture content analysis. Consequently, granules were blended by adding relevant excipients prior to be compressed in Prexima 300, middle size-industrial tablet press that was equipped with Euro-D turret hosting 27 stations and Euro-D oblong biconvex punches 13.5×8.2 mm; standard feeder and paddle with flat profile were used (Table 1).
| PARAMETER | m.u. | VALUE | |||
| Batch name | – | Met 1 | Met 2 | Met 3 | Met 4 |
| Paddle speed | rpm | 20 | 20 | 20 | 20 |
| Pre-compression force (PCF) | kN | 10 | 6.8 | 7 | 7.1 |
| PCF Relative Standard Deviation | % | 2.7 | 2.9 | 3 | 2.5 |
| Compression force (CF) | kN | 15.6 | 12.2 | 12.3 | 12.5 |
| CF Relative Standard Deviation | % | 3.8 | 4 | 3.5 | 3.4 |
| Turret speed | rpm | 20 | 32 | ||
| Production speed | tbl/h | 32,400 | 51,840 | ||
Table 1: tableting process parameter for Prexima 300
At the end, tablets were coated in Perfima Lab equipped with 70 L perforated drum.
Film-coating used was Opadry OY White (Colorcon, UK), prepared at 14 % w/w solids concentration in water to reach a 3 % of weight gain: same recipe was applied at all the batches manufactured.
Based on the results obtained on Prexima 300, tableting output of metformin granules was up-scaled in Prexima 800, a rotary double side tablet press equipped with Euro-B turret hosting 65 stations and B punches size 13.5×8.2 mm: same process parameters optimized in Prexima 300 but different tablet press speed were accomplished.
The machine speed-upfrom 51,840 tbl/h, to 312,000 tbl/h (S1), 429,000 tbl/h (S2) and 507,000 tbl/h (S3, Table 2).
| PARAMETER | m.u. | VALUE | |||||
| Test Code | – | S 1 | S 2 | S 3 | |||
| SIDE | – | 1 | 2 | 1 | 2 | 1 | 2 |
| Paddle speed | rpm | 15 | 25 | 35 | |||
| Pre-compression force (PCF) | kN | 12.4 | 11.8 | 12.4 | 11.9 | 12.1 | 12.6 |
| PCF Relative Standard Deviation | % | 2.7 | 2.6 | 3.5 | 4 | 3.8 | 4.6 |
| Compression force (CF) | kN | 26.5 | 25.5 | 26 | 26 | 25.9 | 26 |
| CF Relative Standard Deviation | % | 2.8 | 2.9 | 3.5 | 4.3 | 4 | 3.8 |
| Turret speed | rpm | 40 | 55 | 65 | |||
| Production speed | tbl/h | 312,000 | 429,000 | 507,000 | |||
Table 2: tableting process parameter for Prexima 800
4. Results and discussions
Powder was characterized from a technological point of view before and after granulation: flowability index (iCarr %) passed from 26% to 14%: this means that granules flow properly and, as expected, flowability increases after the granulation compared to raw materials.
Thanks to smooth and reproducible tableting process, production with Prexima 300 was performed continuously for 40 minutes for each granulation batch. Tablets were checked every 5 minutes in terms of weigh, thickness and strength showing high stability over the time (Figure 1, Figure 2, Figure 3).
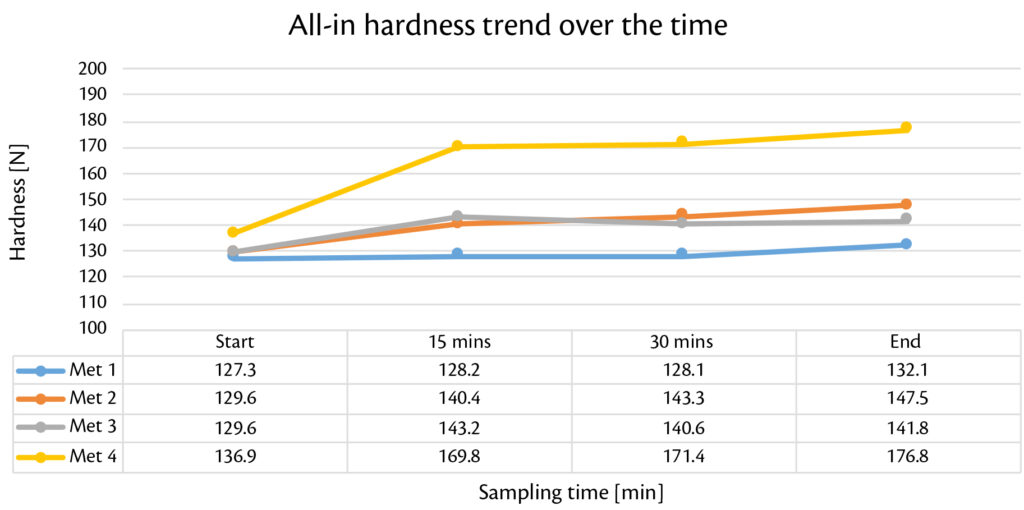
Figure 1: all-in hardness trend over the time for all batches produced with Prexima 300.
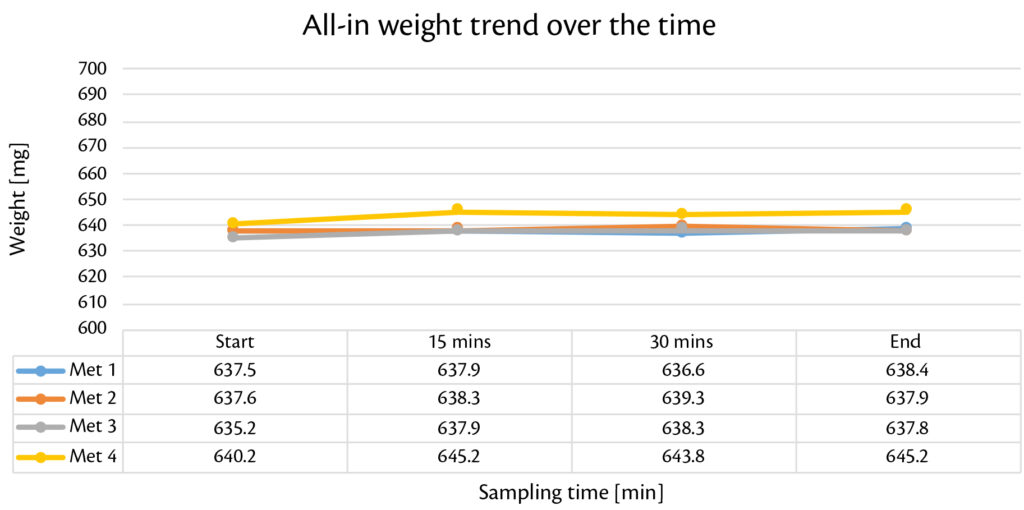
Figure 2: all-in weight trend over the time for all batches produced with Prexima 300.
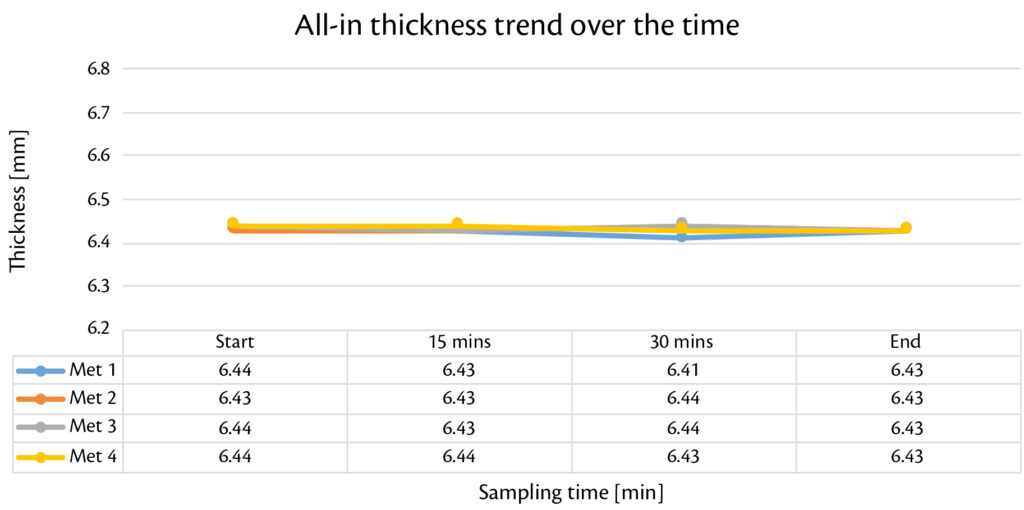
Figure 3: all-in thickness trend over the time for all batches produced with Prexima 300.
Globally, results are within the target limits with very low Relative Standard Deviation (RSD) for each tablets’ technological caharacterization. Weight RSD confinrms this theory: all the valures under 0.5%.
The same approach was considered to evaluate tablets’ quality (Table 3) and process reliability with Prexima 800: tablets were monitored during a continuous production while tablet press output was up-scaled from 40 to 55 and 65 RPM giving always very low Relative Standard Deviation for all the values monitored.
Results were good also at more than 500,000 tbl/h meaning that up-stream processes allows high machinability and tabletability of the API studied.
| PARAMETER | m.u. | VALUES | ||||||
|---|---|---|---|---|---|---|---|---|
| – | S1 | S2 | S3 | |||||
| – | Side 1 | Side 2 | Side 1 | Side 2 | Side 1 | S1de 2 | ||
| Average weight | mg | 697.9 | 697.9 | 691.7 | 692 | 692.3 | 692.5 | |
| RSD | % | 0.36 | 0.45 | 0.69 | 0.64 | 0.87 | 0.87 | |
| Average strength | N | 152.1 | 148.9 | 141.8 | 144.1 | 136.1 | 146 | |
| RSD | % | 5.13 | 6.55 | 8.78 | 8.92 | 14.95 | 6.92 | |
| Average thickness | mm | 5.66 | 5.66 | 5.64 | 5.66 | 5.64 | 5.66 | |
| RSD | % | 0.15 | 0.24 | 0.30 | 0.24 | 0.35 | 0.36 | |
Table 3: tablets’ technological characteristics for Prexima 800 production.
5. Conclusions
Process was clearly optimized from dispensing to final coating: all the steps went smoothly showing stability and reproducibility over the time as well as fulfilling target request thanks to IMA equipment’s flexibility.
Tableting output was extremely increased even thanks to good scale-up from middle-size (Prexima 300) to industrial large-scale machine (Prexima 800) than for the reliability of the up-stream processes.
References
[1] https://www.who.int/news-room/fact-sheets/detail/diabetes
[2] https://medlineplus.gov/druginfo/meds/a696005.html
Paper sections:
Last Submitted Papers
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
