
LYNFINITY: continuous aseptic spray freeze drying. Process, Technology and Product Characterization.
Introduction
Freeze drying, also known as lyophilization, is a process which removes water from sensitive products without damaging them. This process is a method of choice for preserving sensitive products at ambient temperatures that do not require a cold chain commitment. Additionally, freeze dried products can be easily reconstituted in water or saline solution at a point of care administration. Some of the common examples of freeze dried products are antibiotics, bacteria, vaccines and nearly 50% of biopharmaceutical products listed by USFDA and EMA.1 Despite possessing several advantages, conventional freeze drying is almost universally executed as a batch method which results in the several drawbacks listed below:
A. Batch heterogeneity
This includes both heterogeneity in freezing behavior e.g. stochastic ice nucleation leading to variations in the product specific surface area as well as heterogeneity in the heat transfer process e.g. – edge Kv effect leading to variability in drying time and product residual moisture across the shelf.3
B. Process time constraints
Depending upon variables like critical collapse temperature, a batch freeze drying process can be time consuming. In fact, it is not uncommon for a freeze drying cycle to last from two to seven days.
C. Inefficient heat transfer and high energy consumption
A conventional batch freeze drying is an energy intensive process where the majority of energy is consumed by the refrigeration system responsible for shelf and condenser cooling. The heating and cooling of chamber walls is inefficient as well. Additionally, since the area of contact between the bottom of the vial and heat source i.e. shelf surface is limited, the heat transfer in a batch freeze drying process is an inefficient process, typically with a 2-3 % efficiency.
D. Flexibility and Scale up issues
Batch freeze drying typically involves large investment in associated equipment like a vial liquid filling line, capping station that are costly and increase the real estate cost as well. Additionally, since a batch freeze drying cycle is optimized for a particular vial type and fill volume, its upscaling requires full reoptimization and process revalidation resulting in a high production costs.
In order to reduce the limitations of a conventional batch freeze drying process, pharma and biotechnology industry is increasingly focusing on next generation continuous freeze-drying technologies with Spray Freeze Drying (SFD) emerging as one of the most promising candidates. The advantages of a continuous drying process include:
A. Uniform process conditions for the product
Freezing and heating under identical conditions minimizes any variation in product quality. The advantages of spray drying techniques over
a conventional freeze drying processes is due to generation of uniform droplets via vibration assisted spraying process which generates small micro pellets leading to significantly reduced freeze drying time. 4
B. Higher energy transfer efficiency
In a continuous spray freeze drying process, the energy from shelf is directly transmitted to the surface of particles, where the drying front initiates. This heat transfer mechanism eliminates major heat transfer barriers like glass and product cakes having (poor thermal conductivity) that are present in a conventional batch freeze drying process.
C. Reduced manufacturing footprint
From a commercial point of view, a reduction in manufacturing footprint results in lower operational investment and time needed to deploy a new process. Continuous manufacturing can potentially reduce capital expenditures from $1 billion per facility to hundreds of millions to eventually tens of millions and the time for construction from years to months.
Advantages of LYnfinity Technology
LYnfinity technology focuses on bringing to the market a robust yet gentle continuous aseptic process for spray freeze-drying with an eye toward achieving high throughput and high cycle efficiency. The choice of two models viz. a laboratory version and a production version allows for easy model building and cycle development. Some of the key applications of LYnfinity technology are as follows:
1. Antibiotics and other bulk dried products require milling and powder filling today, LYnfinity provides an opportunity to freeze dry product in bulk format and subsequenly powder fill into the final container.
2. Self-administration requires use of pre-filled syringe/cartridge systems. LYnfinity provides an efficient drying technique compared to drying in syringes.
3. Vaccines require high-volume, low-downtime/ low-cost production. A continuous process offered by LYnfinity substantially minimizes downtime.
4. LYnfinity provides significant opportunity to reduce recon time in hard-to-reconstitute products.
LYnfinity Aseptic SFD Process
A. The Spraying Process
The product spray is initiated when a steady laminar product feed is broken into uniform droplets at
the top of the freezing chamber. From the product reservoir, liquid product is fed to a temperature- controlled droplet zone through a specially designed nozzle mounted at the entrance of the freezing chamber. When the laminar liquid jet is mechanically disturbed at a set frequency, droplets of uniform size are formed. Generation of uniform droplets is not only important for consistent drying operation but also for downstream powder filling. Figure 1 shows the imaging of the droplet formation process for a range of upstream parameters as seen using a monochromatic high-speed camera shot at a 2 ms frame rate.
B. The Freezing Process
The stream of liquid droplets is frozen in the freezing column, cooled using a double walled jacket. Liquid nitrogen and silicone oil cooling jackets are utilized around segments of the freezing chamber for temperature control. The gas inside the cylindrical stainless steel cooling chamber is maintained below -130 °C. The liquid product nozzles are mounted directly above the freezing chamber and the freezing column itself is situated above the drying chamber. After conditioning the freezing chamber, the product is sprayed into the chamber through the nozzle mounted at the top of the freezing column. The liquid product freezes as the stream of droplets falls vertically through the column of cooled gas. Rapid freezing allows maintaining the spherical shapes obtained from the spraying process. For example, it is estimated that at a gas temperature of -150 °C and a mean volumetric drop diameter of 500 microns, the drop freezes in less than 5 feet from the point of injection into the cooling chamber. Finally, individual frozen droplets collect at the bottom of the freezing chamber, awaiting transfer to the chamber below. From a product standpoint, rapid freezing of liquid droplets locks their spherical shape and significantly reduce the time available for proteins to aggregate or diffuse into water-air and water-ice interfaces which facilitate denaturation process.6
C. The Drying Process
The drying module is designed for continuous operation by utilizing intermediate vacuum chambers, cascading temperature-controlled vibratory shelves, and a dual ice condenser setup. Following uniform droplet generation and freezing, the frozen product particles are transferred from the freezing chamber to the drying chamber. An intermediate chamber, utilizing a dedicated vacuum pump and jacket cooling, allows for transfer between the freezing chamber at atmospheric pressure and the drying chamber under vacuum without impeding continuous operation. The freeze dryer chamber is a pressure vessel that houses the product shelves. Continuous operation dictates controlled movement that allows sufficient resident time for drying. However, it is imperative that the transport be gentle on the product.
Thus, here the product is moved at a controlled rate through a cascading shelf stack, shown in figure 2, using vibratory agitation for transport. A system of vibratory drives is mounted from the outside of the vessel through vacuum seals into the chamber and is mounted to each pair of shelves. The system is used to vibrate the shelves to move the product through the drying chamber in a gentle, controlled manner. The drying chamber contents are both agitated and heated to promote rapid sublimation and prevent agglomeration.

Figure 1. Uniform droplet generation process in LYnfinity.
Each shelf is designed with a serpentine path of channels through which a heat transfer fluid is passed for temperature control. Heat exchangers,
in series, through which heat transfer fluid is circulated by a pump for each shelf allow for individual shelf temperature control. In traditional freeze dryers, a single ice condenser is defrosted and drained before it can be used again, a process which requires down time. LYnfinity features a dual ice condenser setup allowing continuous operation with condenser change-over during drying.

Figure 2: Particle transport on LYnfinity shelves with lyo-spheres

Figure 3. LYnfinity spray freeze dryer uniform shape and excellent flowability.
Influence of SFD Process on Product
Quality Attributes
A. Particle Size
One of the important potential advantages of SFD process is the flexibility to generate particles of different size ranges which in turn allows some control over the particle density as well. This allows applications ranging from pulmonary drug delivery drug product storage and filling for which flowability is an important quality attribute.

Figure 4. The microparticles generated in LYnfinity SFD showing
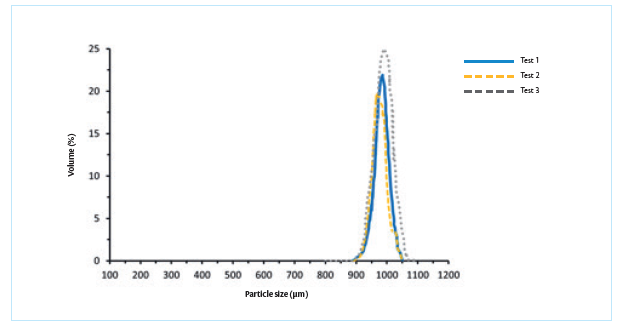
Figure 5. The particle size distribution observed via laser diffraction shows excellent control of particle size around 600 microns. The particle size results generated via laser diffraction and SEM are in good agreement with each other.

Figure 6. SEM micrographs of microparticles generated via LYnfinity SFD showing spherical shape with characteristic abscesses. The SEM image on right shows product morphology obtained via conventional freeze drying.
B. BET-Specific Surface Area (BET-SSA)
The solubility and dissolution rate of a drug is often intrinsically related to its surface area. For example, as the particle becomes smaller, the surface area to volume ratio of that drug increases and the larger surface area allows greater interaction with the solvent, which often results in increased solubility. When performing risk assessment under ICH Q9 guidance, the specific surface area of particular drug product is often considered as a Critical Quality Attribute (CQA) and is frequently included as a part of the overall control strategy for a drug manufacturing process.
One of the key alterations in quality attributes of a SFD product is a significant increase in its SSA as compared to the product obtained via conventional freeze drying. The process of liquid droplet generation followed by solidification via rapid cooling plays a key role in SSA.
| Sample | BET-SSA (m2/g) |
| LYnfinity – SFD | 7.03 |
| Conventional FD | 0.47 |
Table 1. The difference in SSA of a product dried via SFD vs conventional freeze drying can be as high as 15 folds.
Effects of droplet generation conditions and formulation variable on the particle size and stability in a SFD context have been reported with an observation that particle size was inversely related to the specific surface area.8 This observation is important from a product stability standpoint because pharmaceutical stability in solids is not purely driven by thermodynamics.9 In fact, for biological molecules like proteins, stability correlates with the fraction protein residing at the surface of the particle and higher SSA leads to increased protein exposure at the particle surface. This in turn makes surface exposed-proteins more susceptible to the three key chemical degradation processes viz. deamidation, isomerization, and oxidation which in turn, increase the potential for protein aggregation. It is noteworthy that addition of surfactants do not necessarily alter the specific surface area of the formulation but their action is rendered via occupation of the ice interface which partially alleviates protein denaturation at the particle surface.
C. Reconstitution Time
SFD products exhibit similar reconstitution time, if not better, as compared to the conventionally freeze dried products at the cost of slight increase in turbidity or opalescence potentially generated from particle aggregation. SFD technology has allowed more rapid wetting and faster dissolution of poorly water soluble drugs formulated with polyvinyl alcohol and polyvinylpyrrolidone. Enhancement in wetting (from contact angle measurements) and dissolution rates of SFD danazol-poloxamer and carbamazepine-sodium lauryl sulfate particles in comparison to the lyophilized samples or their physical mixtures has already been verified via independent experiments reported in the literature.11 However, such results are not assumed to be universally applicable and therefore, formulation composition as well as the role of surfactants is being compared to a traditional lyophilization process to better understand the reconstitution properties of a SFD product.
It is important to note that each of the following steps in a commercial drying process like spraying (spray drying or spray freeze drying), freezing (except foam drying) and final drying steps may contribute towards aggregates generation. Interestingly, preliminary investigations performed in LYnfinity indicate that increasing the surfactant concentration may help in reduction of aggregation induced opalescence and turbidity. The significance of surfactants in reduction of insoluble protein aggregates have been reported in the literature as well. For example, in one freeze thaw study, freezing of recombinant human anti-IgE monoclonal antibody (rhMAb) formulation (60:40 rhMAb : trehalose) without the use of Tween 20 surfactant resulted in substantial (29%) insoluble aggregates that appeared as white precipitate. Spray-freezing and thawing of same formulation with 0.05 % Tween 20 under the same conditions resulted in formation of only 1% insoluble aggregates, thereby showing the potential importance of surfactants in reduction of opalescence or turbidity associated with the reconstituted spray freeze dried products.
Figure 7. Reconstituted sample (right) showing the characteristics opalescence and turbidity associated with a SFD product. The clearer drug product
control is on left.
Summary
The selection of a specific drying process for a drug product relies significantly on end objectives deemed critical for successful drug application.
The inherent drawbacks associated with traditional drying techniques viz. high production costs or loss of product quality due to their exposure to process-related stresses are making drug manufacturers increasingly gravitate towards the continuous manufacturing. From a business point of view,
it is more important than ever to invest in a drying technology that offers a lower footprint, better process efficiency, more uniform product quality attributes along with superior long term product stability. Spray-freeze drying is at the fore front of such emerging drying technologies, especially for products that will benefit from good particle size control, particle size distribution, powder flowability and yield. It is already proving to be a worthy candidate for improving the bioavailability of pharmaceutical formulations suffering from low water solubility.12 The key lies in meaningful collaboration between technology provider [OEM] and product developers [pharma companies] and LYnfinity is currently playing the role of a much sought after beacon as far as collaborations for the Spray freeze drying technology is concerned.


