
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |IMA Active drives forward the successful uptake of Continuous Manufacturing in the pharmaceutical industry.
Federica Casati, New Pharmaceutical Technologies Manager at IMA Active Guai Bertuzzi, Product Manager for process equipment at IMA Active |
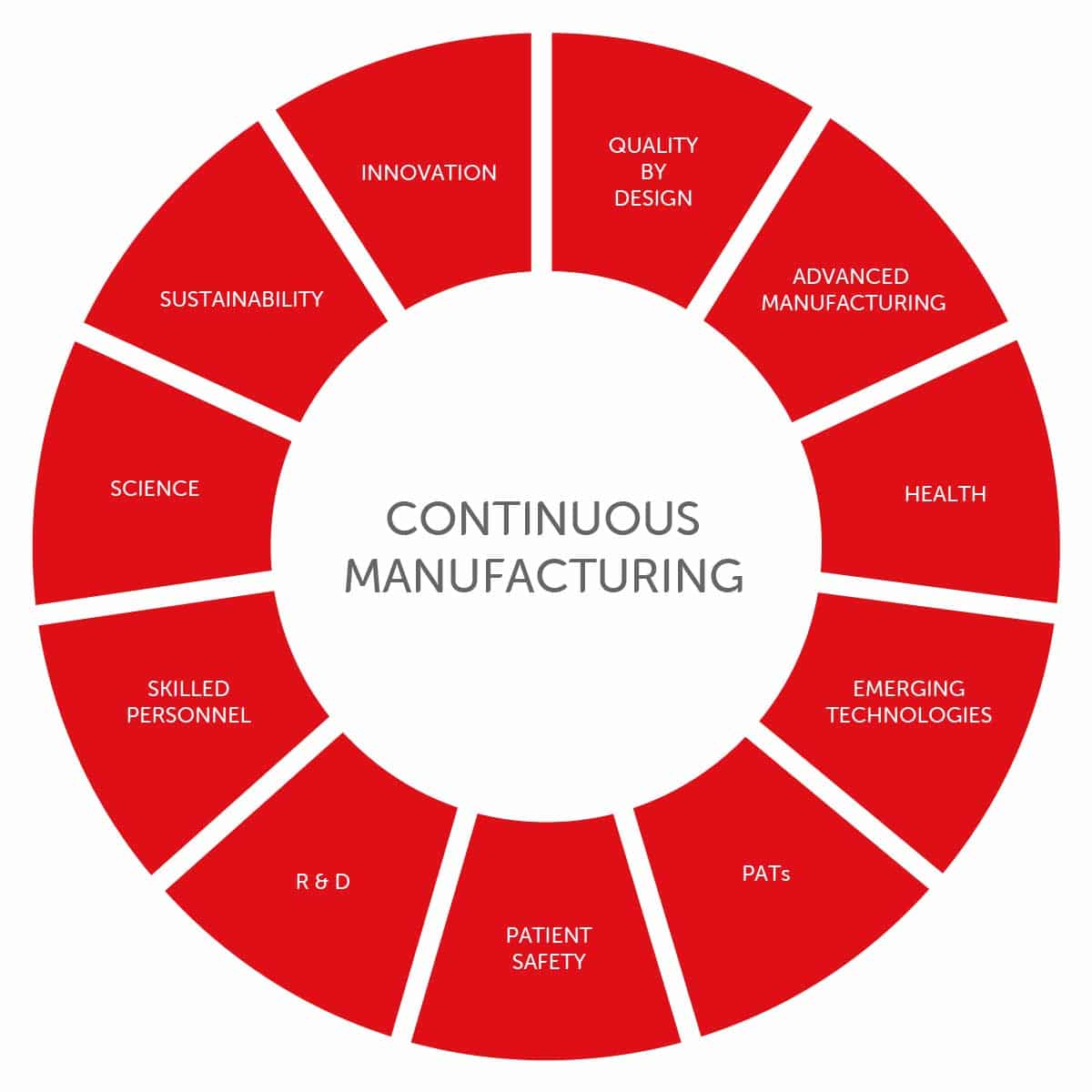
Continuous Manufacturing represents the next generation of pharmaceutical manufacturing processes. Unlike traditional batch processes, which involve several steps, Continuous Manufacturing operates continuously, allowing for real-time control and improved efficiency being totally aligned to the lean manufacturing methodology. IMA Active is committed to supporting pharmaceutical innovation with the intention of driving solid dose manufacturing into an era focused on product quality and process efficiency ensuing higher patient safety.
IMA Active is also strongly engaged in the front line of sustainability within the frame of a corporate commitment to implementing a virtuous and sustainable approach to the future, involving all its stakeholders.
With the program “IMA ZERO: impacting present, affecting future.” IMA Group highlights its awareness that today’s commitment will make the difference in tomorrow’s world.
The Continuous Manufacturing projects meet these objectives through process efficiency improvement and abatement of energy consumption.
IMA’s dedication to technological innovation has always been an element representing a strong competitive advantage for the whole IMA Group: significant, ongoing investment in R&D underpins IMA’s growth path and its ability to create value.
IMA Active is aware that the adoption of Continuous Manufacturing is not simply acquiring the necessary equipment and letting it run, but process control in combination with the proper control strategy is indispensable. Since the adoption of Continuous Manufacturing marks a paradigm shift, in IMA we decided to build and train a top-notch team focusing on process engineering, process analytical technologies (PATs), advanced manufacturing and the related emerging technologies. IMA Active fully embraces the concept that quality cannot be tested on products, but quality is built into pharmaceutical products through a comprehensive understanding of the product and, especially, the manufacturing process. Employing Quality by Design (QbD) principles has become a key feature for pharmaceutical manufacturers to gain valuable insights into the cause-and-effect relationships between process parameters and CQAs (Critical Quality Attributes).
IMA Active has been advocating the development of Continuous Manufacturing and its implementation through two different approaches for many years.
A more disruptive approach consists in the partnership with CONTINUUS Pharmaceuticals, a spin-out of the Novartis-MIT Center for Continuous Manufacturing. CONTINUUS Pharmaceuticals and IMA Active offer breakthrough “end-to-end Integrated Continuous Manufacturing” which enables a seamless process from the synthesis of the active ingredient to the manufacturing of the final dosage form. Advantages of an end-to-end integrated process include bringing the modern manufacturing approach closer to “on demand,” preventing or reducing some of the problems with the current API supply chain.[1],[2],[3],[4],[5]
The second approach to Continuous Manufacturing, closer to conventional manufacturing technologies, includes the Continuous Direct Compression (CDC) and the Continuous Coating (Croma and Accela CTC) (https://ima.it/pharma/continuous-manufacturing/academy/).

Moreover, IMA Active is also promoting Continuous Manufacturing applied to the capsule filling process (Continuous Direct Encapsulation).
The Emerging Technology Program graduated Continuous Direct Compression (CDC) in 2021,[6] and a pharmaceutical company that switched to Continuous Manufacturing reported:
- 50% reduction in operating costs;
- 33% reduction in waste;
- 80% reduction in manufacturing and testing cycle times;
- 66% reduction in time from testing to release;[7]
as CDER’s Perspective publishes in 2023.
An IMA Active Continuous Manufacturing line can produce and coat tablets continuously without the need for an intermediate granulation step. An IMA Continuous Manufacturing line features integrated PAT sensors and is orchestrated by the Maestro integrated control system which are the keys for in-line product quality monitoring, data collection and process control (https://ima.it/pharma/continuous-manufacturing/academy/).
Nowadays, pharmaceutical companies need to fully understand the operational gains converting a traditional batch manufacturing to a continuous-based innovative manufacturing attitude. IMA Active deeply believes that fostering personnel expertise is essential for Continuous Manufacturing’s successful adoption within the pharmaceutical industry. For this reason, IMA Active has a Continuous Manufacturing team at the IMA Active Competence Center whose members are pharmaceutical scientists, process scientists, chemical and process engineers engaged in supporting companies in process understanding, feasibility studies and control strategy so as to keep up with any Continuous Manufacturing advancements.
The Continuous Manufacturing team at the IMA Active Competence Center has been intensively studying the CDC approach considering each process phase and piece of equipment, starting from continuous feeding, continuous blending, tableting, through to coating. In this regard, over recent years the continuous blending advantages, in terms of blend uniformity and final tablet characteristics, have been investigated by the IMA Active Continuous Manufacturing team. Working alongside excipient producers, the impact of raw materials on continuous blending performance and drug product quality have been examined too.[8]
Moreover, continuous monitoring of the blend and tablet uniformity have been consolidated collaborating with PAT manufacturers.
A CDC line works with an NIR probe analysing the content uniformity of the powder blend, either right outside the continuous blender or inside the tablet press feed frame right before the powder blend goes into the die to become a tablet. In addition, the Continuous Manufacturing team at IMA Active has carried out wide studies about residence time distribution, considering the importance of material tracking during continuous processes. As far as continuous coating is concerned, the working principle of both Croma and Accela CTC is based on consistent tablet flow while continuously spraying the liquid film for coating through the drum once the state of control is reached, thereby ensuring uniform product quality.
Because the technoeconomic criteria, products, steps, and scales vary between applications, the IMA Active Continuous Manufacturing team can advise the proper manufacturing solution based on production requirements.
Continuous Manufacturing processes can be:
1) fully end-to-end continuous, including both drug substance and drug product;
2) fully continuous for drug product;
3) hybrid manufacturing of batch and continuous.
The IMA Active Competence Center can support pharmaceutical companies in adopting Continuous Manufacturing with feasibility and process understanding studies while illustrating the benefits of switching from batch manufacturing to Continuous Manufacturing through brainstorming, discussions, and trials with placebo or real products; together with personnel training on equipment, process, and use of PATs.
References
[1] Hu C., Testa C.J., Wu W., Shvedova K., Shen D.E., Sayin R., Halkude B.S., Casati F., Hermant P., Ramnath A., Born S.C., Takizawa B., O’Connor T.F., Yang X., Ramanujam S., Mascia S., An automated modular assembly line for drugs in a miniaturized plant, “Chemical Communications”, 56(7), 1026-1029, 2020.
[2] Hu C., Testa C.J., Born S. C., Wu W., Shvedova K., Sayin R., Halkude B.S., Casati F., Ramnath A., Hermant P., Takizawa B., O’Connor T.F., Yang X., Ramanujam S., Mascia S., E-factor analysis of a pilot plant for end-to-end Integrated Continuous Manufacturing (ICM) of pharmaceuticals, “Green Chemistry”, 22(13), 4350-4356, 2020.
[3] Testa C. J., Hu C., Shvedova K., Wu W., Sayin R., Casati F., Halkude B.S., Hermant P., Shen D.E., Ramnath A., Su Q., Born S.C., Takizawa B., O’Connor T.F., Yang X., Ramanujam S., Mascia S., The design & commercialization of an end-to-end continuous pharmaceutical production process: a pilot plant case study, “Organic Process Research & Development OPR&D”, 24(12), 2874-2889, 2020.
[4] Su Q., Hermant P., Casati F., Halkude B., Wu W., Ramnath A., Dubey A., Born S., Takizawa B., Mascia S., “Model predictive in vitro dissolution testing in pharmaceutical continuous manufacturing: An equivalence study”, AIChE Journal, 2023.
[5] Framework for Regulatory Advanced Manufacturing Evaluation (FRAME) https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cders-framework-regulatory-advanced-manufacturing-evaluation-frame-initiative
[6] News from Emerging Technology Program (ETP)
https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/news-emerging-technology-program-etp
[7] CDER’s Perspective on the Continuous Manufacturing Journey
https://www.fda.gov/media/173811/download?attachment
[8] Hebbink G.A., Janssen P.H.M., Kok J.H., Menarini L., Giatti F., Funaro C., Consoli S.F., Dickhoff B.H.J., Lubricant sensitivity of direct compression grades of Lactose in continuous and batch tableting process, “MDPI Pharmaceutics”, 15(11), 2575, 2023.
[9] IMA Active webinars on Continuous Manufacturing.
https://ima.it/pharma/continuous-manufacturing/academy/
Last Submitted Papers:
- Natural coating formulations: IMA is ready for the latest trend.
- New solutions for Nutraceuticals.
- Component washing: better with less.
- Coating conundrum grappling with minitablet film coating myths.
- Influence of material and capsule filling process with Minima on aerosolization performances by DPIs.
- The potential of Croma continuous coater.
- How to enhance tableting production with a paracetamol based formulation.
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
