
How to monitor HPMC concentration through conductivity measurement.
Pietro Pirera, Product Manager for capsule fillers at IMA Active |
1. Abstract
This work is devoted to optimize the control of concentration of hydro alcoholic solutions of hydroxyl propyl methylcellulose (HPMC, commercially known as Pharmacoat® 603) used, in replacement of gelatin solutions, to seal capsules in the packaging of pharmaceuticals. The control of HPMC concentration is essential to ensure a perfect, uniform and stable sealing of capsules and to avoid counterfeit. In this paper we focus on the measurement of solution concentration. Usually, sealing solutions concentration is monitored by measuring the solution viscosity through a viscometer, as concentration and viscosity are mutually univocally related, through a linear correlation. HPMC solutions, however, behave as pseudoplastic, rather than Newtonian, fluids, thus their viscosity depends not only on the solution concentration, but also on the shear rate applied during stirring and/or transfer of solution from one container to another, and a viscosity measurement is not sufficient to determine their concentration.
In this work we demonstrated, after a comprehensive experimental analysis in the laboratory and in the field with Hermetica sealing machine, that a proper and univocal measure of HPMC solution concentration is its electrical conductivity. In particular, it was observed that concentration is much better related, through a linear correlation, to the solution conductivity rather than to its viscosity. The correlation between concentration and conductivity was tested in the concentration range 12-25% and a mathematical expression was proposed for it. Such correlation allows to control and adjust the solution water/alcohol ratio with a simple measurement such as the conductivity one.

2. Introduction
The capsule sealing process is primarily targeted to avoid the counterfeit: a sealed capsule can be opened only by breaking it in two parts. Therefore, it is impossible to replace its content without compromising the capsule integrity.
The sealing of capsules is performed by applying a solution of gelatin or hydroxyl propyl methylcellulose (HPMC, commercially known as Pharmacoat® 603) on the junction between the capsule body and cap. While the sealing with gelatin solution has been used successfully for years, the sealing with HPMC is relatively new.
HPMC capsules have some advantages respect to the gelatin capsules:
• Lower and stable amount of moisture.
• Higher reproducibility of the manufacturing process.
• Better customer acceptability: gelatin is of animal origin while HPMC is obtained from vegetals.
• Improved release profile of some APIs.
An important part of the capsule sealing is to maintain constant the concentration, and therefore the viscosity, of the solution to be applied on the capsules.
In case of gelatin, the concentration is monitored with a viscosimeter. The water evaporation from the solution (which is heated to 40°C) causes a linear increase of the viscosity. When this happens, more water is dropped in the tank with the solution.
In case of HPMC, the solution is not aqueous but hydroalcoholic. The viscosity of HPMC solution depends not only from its concentration, but also from the mechanical stress to which it is subjected.
The solution behavior under force application (like stirring, or simply transferring it from one container to another) can be different.
The HPMC solutions are pseudoplastic, meaning that their viscosity decreases with increasing shear rate, as opposed to the Newtonian fluids (like gelatin solution) which maintain constant viscosity with the shear rate. Since the results obtained with the viscosimeter were not reproducible, it was decided to control the concentration by measuring the electrical conductivity of the solution, supposing the HPMC is charged. Therefore, the higher the concentration, the higher the conductivity.
This idea was tested by measuring the conductivity of HPMC solution, starting from 25% concentration and by diluting it with solvent until lower concentrations of 12%.
This method is obviously off line and time consuming, but it was useful to understand whether there was correlation between conductivity and concentration. After the laboratory tests we passed to use the instrument in Hermetica sealing machine during trials with customer.
The conductivity value proved to be more related to the concentration rather than the viscosity (which had much higher range of variation). The conductivity value fell between 440 and 520 microSiemens/cm. We also tried to define an equation to describe the relation between the concentration and the conductivity. This relation was linear of the type y (concentration% ) = mx (conductivity )+q.
3. Materials
• Pharmacoat 603 (Shin-Etsu Chemical)
• Ethanol 95%
• Demineralized water
Pharmacoat® 603 (Shin-Etsu Chemical)
4. Instruments and machines
• Hermetica 100
• Heated vessel of 30 L
• Conductivity meter mod. COND 7
• Viscometer Sofraser Sofast BV
• Viscometer Sofraser MIVI 9600
• Thermal moisture analyzer Mettler Toledo HB43-S
5. Machine description
The machine is composed by the loading hopper, for capsules feeding, the sealing group, characterised by sealing baths and sealing discs for the application of the ring on the capsules, the drying group in connection with the ventilation unit through rigid pipe, and finally by the sealing solution preparation group containing control instruments for viscosity and electrical conductibility. Because of ethanol presence in the solution and the consequent development of fumes, sealing, solution preparation and part of drying groups are made according to ATEX regulations.
6. Sealing process
Purpose of the process is to apply a sealing ring to the capsules. After loading the capsules into the feed hopper, these are aligned in specific cavities where the central part is bottomless to receive the banding solution, that is taken from the bottom of the vessel using peristaltic pumps and transferred in the sealing baths. Another group of peristaltic pumps removes the excess solution from the sealing baths and sends it back to the vessel: consequently the solution is subjected to recirculation. In the sealing baths there are sealing discs that apply the ring in the central part of the capsules.
The capsules are then transferred in the drying box and, after solvent evaporation, they are ejected from the discharge hopper.
Concentration control with viscometer has not provided good results due to the imprecision and the non-reproducibility of the measurements. The reason is the stratification of the solution, however trying to reduce this phenomenon by stirring the product, it causes repercussions on the vibration sensor that needs a motionless system.
Therefore, controlling the concentration through electrical conductivity measurements, has been considered a valid alternative.
7. Laboratory tests
Several tests were made to find a correlation between concentration and electrical conductivity.
In the range of interest (12-25% of HPMC) the sealing solution was gradually diluted, so concentration and conductibility values were recorded for every dilution.
A refilling solution, 50:50 w/w water and ethanol, was used to contrast solvent evaporation during the sealing process. For concentration evaluation a thermal analyzer is used: 500 mg of the solution are deposited on the weighing plate and heated to 200°C.
The instrument returns the percentage of moisture lost to evaporation, which, subtracted from 100% gives the percentage content of HPMC (dry residue).
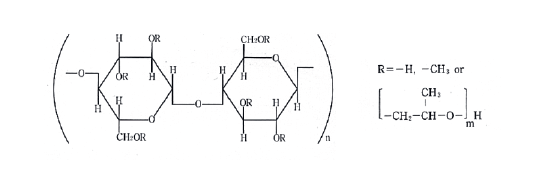
Trend of the concentration as a function of the electrical conductivity of a 25% solution of HPMC
From the figure the trend of the concentration with the conductibility is growing. Slight deviations from linearity can be attributed to interactions between the polymer chains and the solvent.
8. Experimental description – results
Compared to static tests of the laboratory, the sealing process, conducted in the machine, is more complex because of the recirculation factor of the product. It causes an increasing of the evaporation surface. Furthermore due to the presence in the refilling solution of water and ethanol and of their different evaporation rate, in time there is a slight increase in conductivity, despite the concentration remains constant, thanks to the higher conductivity of the water. So considered a generic test “1” as a reference, the trend line obtained has been employed as line of first attempt.
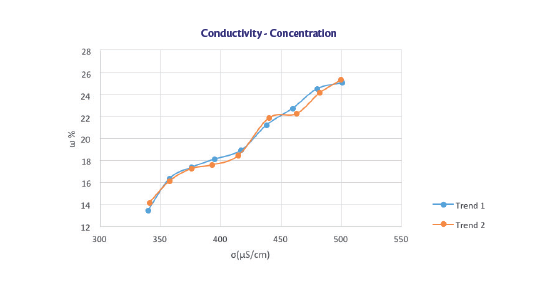
TEST 1

Trend of the concentration as a function of the electrical conductivity of a 25% solution of HPMC
The line [y = 0.0259x + 7.2948] has been used to predict the concentrations of the next tests and, as the following figure shows, the angular coefficient can approximate the experimental data, while the intercept identification is not so immediate.
Comparison between experimental and theoretical concentrations
To optimize the intercept value the standard deviation, Σ (ω sp – ω teo) ^ 2, has been minimized through generalized reduced gradient method to have the minimum difference between experimental and theoretical concentrations. The angular coefficient is the general trend of the concentration with the conductivity, while the intercept depends on all those factors that vary during the sealing: temperature, evaporation rate, mixing rate, different water-ethanol ratio in time.
Since during sealing tests the concentration remains constant, the conductivity varies in a range between 440 and 520 μS/cm and at 20% of HPMC the conductibility is on average 480 μS/cm, the intercept value obtained is 7.568. Here the equation of the line found.
y = 0.0259x + 7.568
The line represents the equation to control average the concentration of the polymer during the sealing of capsules based on electrical conductivity measurements.
In the case of gelatin sealing the refilling solution is added when the viscosity exceeds a threshold value which remains constant in time, in HPMC sealing instead, due to the different water-ethanol ratio in time, the conductivity threshold value increases by about 10 mS/cm per hour.
9. Additional data
Another set of data was obtained from a sealing trial performed on capsules supplied by a Customer.
Due to the specific request, the refilling was made not in the sealing solution tank but in the sealing baths, which were exposed to the atmosphere with a consequent increase in the evaporation rate and greater difficulty in maintaining concentration constant. Below can be seen table and graph of the test.
|
Time |
Conductivity |
Concentration |
Temperature |
Estimated concentration |
Conductivity (stirring) |
Estimated concentration (stirring) |
| min | μS/cm | %w | °C | %w | μS/cm | %w |
| 10 | 435 | 19 | 21,3 | 18,8345 | 485 | 20,1295 |
| 20 | 430 | 19,5 | 21 | 18,187 | 480 | 20 |
| 30 | 410 | 19,5 | 18,8 | 18,187 | 460 | 19,482 |
| 40 | 422 | 20,3 | 19,2 | 18,4978 | 472 | 19,7928 |
| 50 | 430 | 19,6 | 18,6 | 18,705 | 480 | 20 |
| 60 | 435 | 20,7 | 19,6 | 18,8345 | 485 | 20,1295 |
| 70 | 425 | 20,5 | 19 | 18,5755 | 475 | 19,8705 |
| 80 | 430 | 20,8 | 18 | 18,705 | 480 | 20 |
| 90 | 435 | 21 | 18,2 | 18,8345 | 485 | 20,1295 |
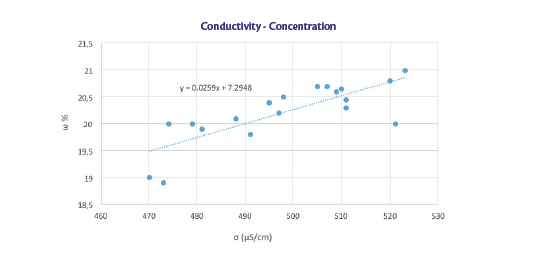
As can be note from the graph, the data obtained were higher than those estimated theoretically from the equation (red and blue curve).
The trend is parallel to the theoretical one, except for the outliers (black and orange circle) that were observed immediately after diluting manually the solution.
The stirring plays an important role in keeping uniform the solution. If left static, the values can differ due to the formation of layers of different concentration.
Therefore, in the sealing baths, where there is no agitation, the values tend to be somewhat lower if compared to the tank where the solution is under constant mild stirring (data from previous tests). If a correction of approximately 50 uS/cm is applied, it can be seen that the two trends (theoretically calculated and the experimental data with the correction, green and purple curve) are more similar. This correction doesn’t influence the slope but the intercept of the equation, which is the only factor influenced by experimental conditions (temperature and stirring rate).
10. Conclusions
The relation obtained represents the more accurate description reached, however other tests are necessary to definitively verify the reliability. It is also required further check of the water-ethanol ratio control during the sealing process in order to get the polymer concentration the only variable affecting the conductivity.
