
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Croma continuous coater: saving energy and process time by using high solids coating material.
Jola Gorreja, R&D Process Engineer at IMA Active Guia Bertuzzi, Product Manager for process equipment at IMA Active |
1. Introduction
The modern pharmaceutical industry is facing the challenge of transition from a traditional manufacturing approach based on batch production to Continuous Manufacturing.
A typical Continuous Manufacturing scenario allows for an increase or decrease in product throughput to meet planned production targets and thereby match market demand.
Croma continuous coater brings innovative, technological progress in manufacturing based on modularity and truly continuous film-coating of tablets. [1-4]
2. Purpose
The objective of this study is to evaluate the application of a modern coating material to use with a high concentration of solids working with Croma continuous coater configured with a single process module.
Nowadays it is possible to find on the market a number of coating systems, ready to use, which help to coat tablets faster, maintaining the same final tablet quality due to the high concentration of solids in the liquid suspension and low viscosity. These new coating systems are robust, able to withstand minor variations of process conditions and guarantee smooth surface appearance as with traditional formulations. Coating trials using different concentrations of solids in suspension have been performed to evaluate both energy and process time saving. The final appearance of the coated tablets has been evaluated by visual inspection and intra- tablet coating uniformity has been measured using ΔE method. [5]
3. Materials and methods
Uncoated placebo oblong tablets (Table 1) have been coated with Opadry QX RED 321A250011 by Colorcon. Opadry QX is a film coating ingredient designed for use in tablet coating processes up to 35% w/w concentration of solids in water, exhibiting a viscosity that can be easily managed in standard spray coating equipment.
| Shape | Oblong |
| Dimensions (mm) | 17 x 7 |
| Excipients | Lactose Mg-Stearate AL2O3 Cellulose in powder |
| Average hardness (N) | 140 |
| Bulk density (kg/m3) | 770 |
| Average weight (mg) | 600 |
Table 1: Placebo tablets.
4. Equipment and process
The coating trials are carried out using a single-module Croma coater in continuous mode operation (Figure 1).

Figure 1: Croma continuous coater, single module.
Uncoated tablets are fed and mixed as they continuously flow through the perforated rotating drum. As tablets pass through the length of the drum, they are heated by process air and at the same time coated by means of liquid film sprayed by a train of guns (Figure 2 and 3).

Figure 2: Continuous coating process scheme.

Figure 3: Continuous coating in Croma.
A single Croma module can reach a tablet throughput in the range of 20-90 kg/h depending on:
• Target weight gain
• % of polymer in suspension
• Tablet shape, dimensions and weight
Therefore, production throughput depends on the spray rate of the coating suspension which in turn depends on the heating capacity of the system, on the polymer nature and the physical and chemical properties of the tablets.
For a given target of weight gain, and once the percentage of solids in the coating suspension are known, it is possible to calculate continuous coater throughput, according to the following formula:

5. Experimental
The film coating suspension of Opadry QX is prepared at 20%, 25%, 30%, 35% of solids concentration (w/w) in water.
Coating trials are performed in continuous mode to achieve a final target weight gain of 3% w/w.
2 sets of trials have been carried out:
• At a constant throughput of 60 kg/h and different concentration levels of polymer suspension to evaluate energy consumption.
• At a constant spray rate of 150 g/min and different concentration levels of coating suspension to evaluate process time.
An increase of concentration of coating materials means a decrease of spray rate once target weight gain and throughput are known. Increasing solids concentration in the coating suspension also means less water to evaporate. Therefore, to maintain the usual suitable temperature 42-44 °C of the tablet bed, it is possible to work at lower inlet air temperature as shown in Table 2.
| # Run | Opadry QX Concentration (%) | Theoretical weight gain (%) |
Tablets throughput (kg/h) |
Spray rate (g/min) |
Inlet air Temperature (°C) |
| 1 | 20 | 3 | 60 | 150 | 75 |
| 2a | 25 | 3 | 60 | 120 | 72 |
| 3a | 30 | 3 | 60 | 100 | 70 |
| 4a | 35 | 3 | 60 | 86 | 67 |
Table 2: Parameters for set 1 of coating trials.
An increase in concentration of the coating suspension means an increase in tablet throughput and a decrease in tablet residence time once target weight gain and spray rate are known. Indeed, it is possible to make the process run faster and achieve higher performance while maintaining the same quality (Table 3).
| # Run | Opadry QX Concentration (%) |
Theoretical weight gain (%) |
Tablets throughput (kg/h) |
Spray rate |
Inlet air Temperature (°C) |
| 1 | 20 | 3 | 150 | 60 | 67 |
| 2b | 25 | 3 | 150 | 75 | 5.4 |
| 3b | 30 | 3 | 150 | 90 | 45 |
| 3b | 35 | 3 | 150 | 105 | 4 |
Table 3: Parameters for set 2 of coating trials.
6. Results
During each run, samples of final coated tablets are taken every 10 minutes and colour uniformity for each sample is evaluated using a portable colour spectrophotometer (ColorEye XTH, X-Rite Corporated). The data are analysed using the (CIE) L* a* b* method. [6] For Opadry QX RED 321A250011, a DE NMT 2,5 is suggested by Colorcon in the product specification sheet [7].
For set 1 of trials, at each solid concentration level, the energy necessary to coat the tablet in continuous manufacturing has been estimated.
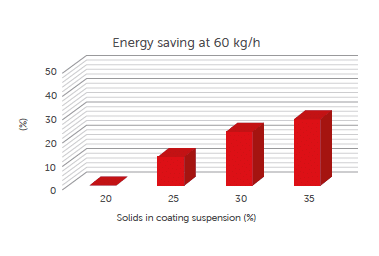
Figure 3: energy saving: comparing different Croma continuous processes with the same tablet throughput but different concentrations of solids in the coating suspension.
Energy consumption has been compared with the one required by the process when using a traditional aesthetic coating suspension, PVA based, at 20% of solid in solution (Run 1).
Moreover, the ΔE values at different concentrations of solids and same tablet throughput are shown Figure 4.
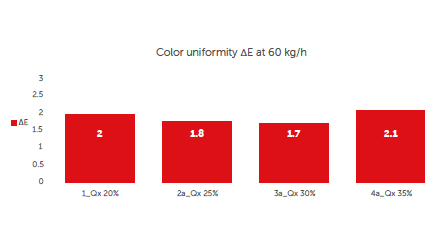
Figure 4: colour uniformity: comparing different Croma continuous processes at constant throughput and different concentrations of solids in the coating suspension.
For the set 2 of trials, ΔE at each throughput has been measured to understand if there is a colour uniformity deviation due to the increase of tablet throughput and consequent decrease of the time that the tablets spend inside the coater (residence time).
Values of ΔE at different tablet throughputs but constant spray rate can be shown in Figure 5.
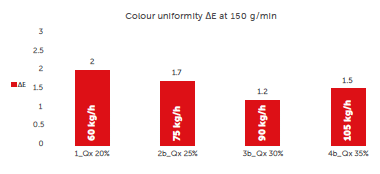
Figure 5: colour uniformity: comparing different Croma continuous processes at constant spray rate and different concentrations of solids in the coating suspension.
Results show that excellent colour uniformity is achieved for all the solids concentration levels of coating suspension and tablet throughputs. However, the runs (3a, 3b) with a 30% w/w of solids concentration provide outstanding performance either in terms of intra – tablet coating uniformity or final appearance of the tablet surface Figure 6.

Figure 6: Tablets at 30% Opadry QX
Conclusion
Results show that Croma continuous coater efficiency is enhanced by the use of coating suspensions at high concentration levels of solids especially when high tablet throughputs are required considering also the advantage of lower energy consumption.
7. Conclusion
Results show that Croma continuous coater efficiency is enhanced by the use of coating suspensions at high concentration levels of solids especially when high tablet throughputs are required considering also the advantage of lower energy consumption.
Paper Sections:
Last Submitted Papers:
- Influence of material and capsule filling process with Minima on aerosolization performances by DPIs.
- The potential of Croma continuous coater.
- How to step-up metformin tablets production from a pilot scale coater to three different industrial scale equipment.
- How to enhance tableting production with a paracetamol based formulation.
- Metformin manufacturing: scale-up from middle-size to large-size rotary tablet press.
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
