
The decontamination cycle with vaporised hydrogen peroxide (VPHP) in isolated filling lines: aspects of development, validation and criticality – Part 1
IsoTech Lab - In-House Isolator Research & Development Laboratory
Introduction
The main objective of an aseptic process is to maintain product sterility by controlling and containing the risk of microbiological contamination.
The use of isolator technology significantly reduces this risk as it confines the process within a specific working environment, an area characterised by extremely limited human intervention and by defined and controlled environmental conditions, obtained through the use of various systems such as microbial retention filters, automatic sterilisation or decontamination processes and prevention of re-contamination from the external environment.
The phases of the decontamination cycle with VPHP
The decontamination of surfaces inside the isolator occurs through the use of a sanitising and sporicidal agent which, in most Pharma guidelines, is recognised in Vapour Phase Hydrogen Peroxide (VPHP).
The vaporised hydrogen peroxide registered at the Environmental Protection Agency (EPA) as a sterilising agent offers numerous other advantages, especially if contained in a chamber below the condensation point, since it:
- reaches all surfaces and volumes to be treated;
- has high sporicidal capacity at very low concentration levels (typically 0.5-2.0 mg/l);
- does not release toxic residue given its spontaneous decomposition into oxygen and water;
- is easy to find on the market at a reasonably low cost;
- can be used in various temperature and relative humidity conditions;
- does not need critical pressure levels, so can be used to decontaminate large volumes
The decontamination process with VPHP inside an isolator envisages two main decontamination mechanisms: dry and wet. For the dry cycle, the concentration of H2O2 is kept below the dew point, whereas in the wet cycle, a quantity of vaporised solution is injected to create an oversaturation of the air in the chamber.
The dry decontamination cycle that characterizes IMA isolators is generally made up of four phases:
- Dehumidification
- Conditioning
- Decontamination
- Aeration
Figure 1 shows the theoretical pattern of VPHP concentration and relative humidity (RH%) during the various phases of a dry cycle
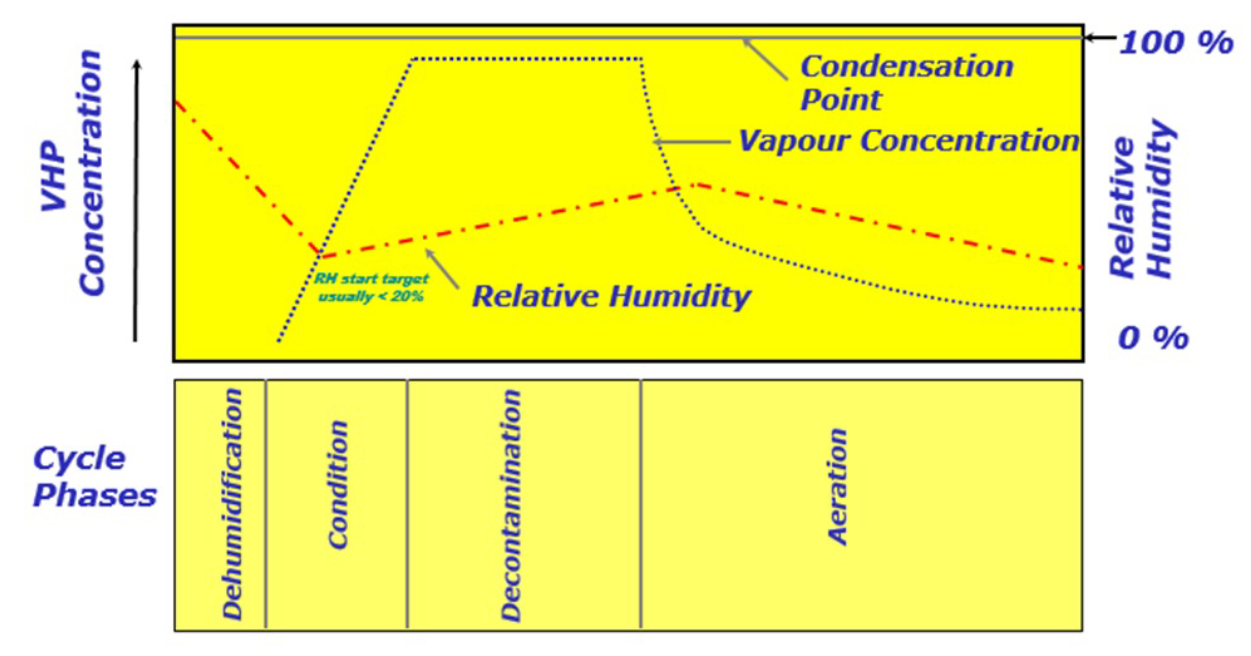
Figure 1- Theoretical pattern of VPHP concentration and RH% throughout the cycle
Let’s take a look at the four phases in detail.
Dehumidification
The purpose of the dehumidification phase is to reach determined temperature and relative humidity conditions inside the isolator and VPHP generator (warm-up phase), before injecting the VPHP in order to achieve the maximum concentration of hydrogen peroxide in a uniform and repeatable way.
Factors such as isolator dimensions, temperature and relative humidity can influence the duration of the dehumidification phase. Therefore, at the line design stage, it is important to appropriately size the HVAC that serves the isolator or systems supplying air to the generator (compressed air or the like).
Conditioning
During the conditioning phase, the solution of H2O2 [35%] is completely vaporised through the “flash vaporisation” process and injected into the isolator chamber until a uniform concentration level is achieved in all areas.
During the Hydrogen Peroxide Vapour injection, the RH% (relative humidity) of the air in the chamber rises.
Decontamination
Once the desired concentration of VPHP in the chamber at the end of the conditioning phase is reached, this concentration must be maintained for a determined length of time, known as “killing time”, important for reducing the microbial concentration.
Aeration
The aeration phase guarantees VPHP removal from the air and the surfaces inside the isolator before production resumes, and features various stages characterised by specific temperature and relative humidity set-points.
It may be effective to split the aeration phase into two stages:
- The initial stage, in which hot dry air is injected into the isolator to encourage the purging of the system, which goes from the VPHP generator to the isolator but, above all, the desorption of VPHP from materials. Indeed, as the temperature rises, the diffusion coefficient of a material increases, encouraging the exchange between the material and the environment. One of the factors that can influence the duration of the hot stage is the relative volatility between H2O and H2O2. Indeed, the water present as a component of the hydrogen peroxide vapour tends to evaporate at lower temperatures than the peroxide component, leading to a considerable increase in the concentration of the latter in the condensation.
This would explain why in the wet process, following the condensation of the hydrogen peroxide during the injection phases, the time required for subsequent desorption of the hydrogen peroxide from the surfaces could take longer.
- A second stage, in which cooler air is injected in order to stop the degassing of materials and thereby accelerate the removal of hydrogen peroxide residue to the required safety limit.
The aeration phase also allows the temperature and relative humidity conditions required for production to be reached.
Figure 2 shows the graph of a real cycle.
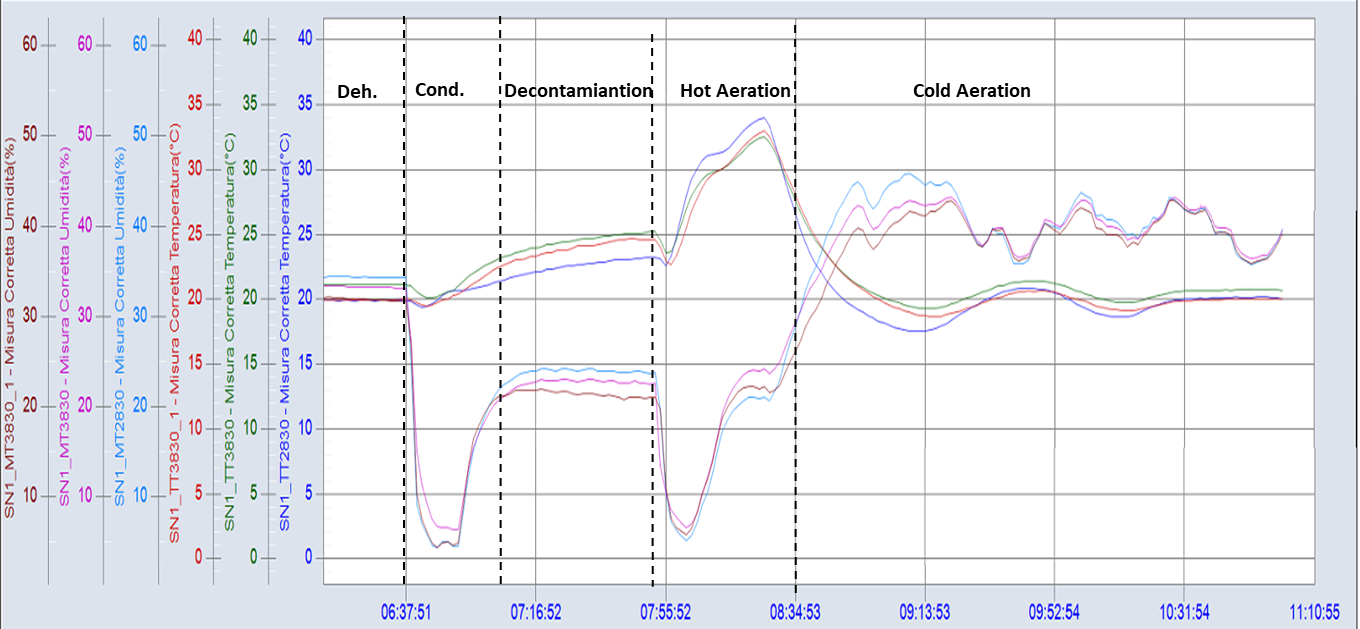
Figure 2 – Graph of main parameters monitored in a real decontamination cycle. Temperature (red), humidity (green), high concentration (dark blue) and low concentration hydrogen peroxide monitoring (light blue) in a filling isolator
Factors that influence the decontamination process
The characterisation and effectiveness of the decontamination process depend on a number of factors, such as:
- Relative humidity (RH%) and temperature of the air;
- Temperature of the surfaces;
Relative humidity (RH%) and temperature of the air
The relative humidity percentage expresses the relationship between the quantity of water vapour contained in an air mass and the maximum quantity that it can contain under the same temperature and pressure conditions:
RH% = PH20 X 100
P*H20 (T)
where PH20 is the partial pressure of water in the air and P*H20 (T) is the tension of water vapour at the considered temperature. If a water-hydrogen peroxide solution is present, its equilibrium conditions modify the parameters contained in the definition of relative humidity, in accordance with Raoult’s law. Taking that into account, it is possible to use the relative humidity measurement to define how far an air mass containing the water-hydrogen peroxide solution in vapour phase is from saturation.
Temperature of the surfaces
The temperature of the surfaces is generally monitored in the first phases of the cycle. This factor is particularly important in a dry cycle, as it can help identify the most critical positions inside the isolator. In general, these positions correspond to the points at which the temperature is lowest and highest: the lowest temperature can correspond to a point at which local condensation could occur, whereas the highest temperature is where the air could be less saturated than at other points.
Condensation
As introduced in previous sections, there are two main decontamination mechanisms with VPHP: dry and wet. The difference between them lies in the desired quantity of condensation during injection into the isolator.
However, with the two types having the same decontaminating effect, the dry cycle provides greater advantages, such as:
- use of minimal concentrations with the same sporicidal effectiveness (1 mg/l compared to 400 mg/l in liquid) with considerably lower costs and consumption;
- rapid, effective removal from the chamber at the end of the decontamination time, thus reducing cycle duration;
- longer life of materials due to less condensation;
- homogeneous distribution of hydrogen peroxide inside the isolator.
All the above factors depend on the thermo-hygrometric conditions inside and outside the isolator (e.g. temperature of isolator glass or air ducts). It is therefore essential to verify the factors under real and final use conditions.
Next issue (Part 2) will focus on decontamination cycle best practices.

Figure 3: VPHP cycle development


