
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Powder layering in a solid wall pan for the fast preparation of sugar spheres.
|
|
1. Introduction
Sugar spheres (also called neutral pellets, nonpareil seeds, microgranules or sugar beads) are inert cores mainly based on sucrose and starch, used as excipients for sustained-release oral solid dose formulations. The aim is normally to coat the sugar spheres with the active ingredient in layers to a maximum of 75%; [1] the properties that have been identified as critical to successful drug loading are surface roughness and sphericity, particle size and its distribution, density, friability and hardness. [2] As reported in the USP and EP harmonized monographs, sugar spheres mainly consist of sucrose and starch and may contain color additives permitted by the FDA for use in drugs.
The two technologies that are mainly used for the preparation of sugar spheres both start with raw sugar crystals: sucrose layering as a liquid syrup (liquid layering) and sugar powder layering (powder layering). In the first case the process is longer but the use of syrup ensures a smooth surface smoothness and spherical shape. By using the powder layering method, process time can be more cost effective, however, great attention must be paid to the parameters in order to obtain a regular shape.
2. Aim of the study
The aim of the study was to evaluate the possibility of manufacturing sugar spheres in a pilot solid wall pan by using the powder layering method whilst also obtaining spheres with good technological properties.
3. Materials, equipment and methods
Sugar crystals (10 kg, particle size corresponding to 90% lower than 200 micron) were used as starting material in all trials. Two batches of sugar spheres were manufactured by liquid layering in the same equipment (GS HP 25, IMA). Liquid layering was performed by repeating a cycle of spray, pause and drying several times; during the spray step a syrup made of sucrose 52.3%, corn starch 22% and water 25.7% was employed. The syrup was kept at 65°C for the entire process.
Powder layering provided several repetitions of spray, pause, powder, pause and drying; the same syrup composition as the liquid layering (same T°) was sprayed as a binder while micronized sugar powder was applied using a screw feeder without the need of any flowability enhancer excipients.
For both methodologies the end of the process was individuated when the target sugar sphere particle size and a loss on drying of less than 4% was reached. Loss On Drying was determined by using a Thermo-Balance (HB43-S-Halogen Moisture Analyzer, Mettler). Morphology was evaluated by Scanning Electron Microscopy (Hitachi TM 3000). Particle Size Distribution was performed by sieve analysis using a vibrating sieve (IG1 S Giuliani) and six standard sieves in the range 100–1000 micron. Friability was tested by using a TA 20 friabilator (Erweka). Flowability was measured by calculating Carr Index obtained after measuring the bulk and tapped density by using SVM 12 (Erweka).
4. Results and discussion
The liquid layering trials provided, as expected, spherical sugar spheres with good technological characteristics but the process time to pass from raw sugar to a final product having d90 between 300 and 800 microns was approximately 16 hours. The first trials, applying sucrose in powder, obtained sugar spheres the same size as those of the liquid layering process and shortened the process time to 3 hours; however, the process did not result in sugar spheres with acceptable sphericity and smooth surface (Figure 1).
Figure 1: morphology of sugar spheres obtained by powder layering before process optimization.
The subsequent experiments focused on optimising the process parameters (in particular spray quantity, pause time, powder quantity, core temperature and pan speed) in order to improve sugar sphere morphology. Table 1 reports operative parameters before and after optimisation of powder layering process.
| u.m. | Before optimization | After optimization | |
| Inlet air quantity | m3/h | 180 | 180 |
| Inlet air T° | °C | 75 | 70 |
| Pan neg. pressure | Pa | -10 | -10 |
| Cores T° | °C | 40 – 44 | 35 – 38 |
| Pan speed | rpm | 18 | 22 |
| Spray/recycling pressure | bar | 1 – 0.8 | 1 – 0.8 |
| Spray time | sec | 10 – 30 | 7 – 21 |
| Spray rate | g/sec | 8 | 8 |
| Powder addition time | sec | 12 – 40 | 7 – 36 |
| Powder delivery rate | g/sec | 16 | 16 |
| Spray and powder | sec | 15 – 45 | 30 – 60 |
| Drying time | sec | 60 – 90 | 60 – 90 |
Table 1: operating parameters before and after process optimisation.
The sugar spheres obtained had a spherical shape and smooth surface (Figure 2 and Figure 3).
Moreover, all the batches produced by powder layering had good yield (higher than 96%), friability below 1% and Carr Index always below 10% indicating excellent flowability.

Figure 2: morphology of sugar spheres obtained by powder layering after process optimization.

Figure 3: cross section of sugar spheres obtained by powder layering after process optimization.

Figure 4: morphology of raw sugar crystals.
5. Conclusion
The results presented in the study demonstrated that sugar powder layering in a solid wall pan is a suitable technology to manufacture sugar spheres with a particle size distribution similar to those obtained by the traditional liquid layering technology (Figure 5) with ideal morphology and technological properties for the subsequent drug layering. The process time could be significantly decreased from 16 to 3 hours.
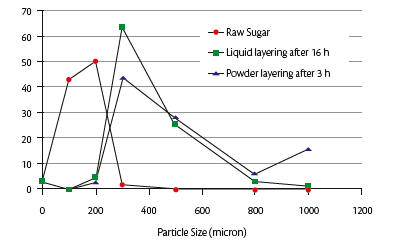
Figure 5: particle size distribution.
References
[1] Werner D., Sugar spheres: a versatile excipient for oral pellet medications with modified release kinetics, “Pharmaceutical Technology Europe”, pp. 35-41, April 2006.. [2] SureSpheres Colorcon Application Data.
Paper Sections:
Last Submitted Papers:
- Influence of material and capsule filling process with Minima on aerosolization performances by DPIs.
- The potential of Croma continuous coater.
- How to step-up metformin tablets production from a pilot scale coater to three different industrial scale equipment.
- How to enhance tableting production with a paracetamol based formulation.
- Metformin manufacturing: scale-up from middle-size to large-size rotary tablet press.
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions

