
Matrix minitablets produced by direct compression on Prexima 80: influence of operative and formulative parameters
Federica Giatti, Compression Technologist at IMA Active Fabriano Ferrini, Product Manager for tablet presses at IMA Active |
1. Introduction
Minitablets are multiple unit dosage forms presenting all the advantages of these systems over the single unit dosage forms such as low risk of dose dumping, high degree of dispersion in the GI tract and reproducible bioavailability. Moreover, if they are manufactured by direct compression instead of granulation or extrusion and spheronization, the production is one step and leads to lower costs and higher production yields [1, 2].

2. Aim of the study
The aim of the study was first to identify the best manufacturing parameters (die feeder speed, turret speed, pre-compression force, range of possible compression forces) to produce minitablets with technological characteristics according to European Pharmacopoeia (uniformity of mass, crushing strength, friability). Subsequently the effect of different cellulose type, molecular weight, particle size and quantity at two different compression forces (Table 1) on technological characteristics (crushing strength, friability) and Theophylline release was evaluated.
| Batch | TH (%) | Cellulose (%) | Cellulose Type | Compression force (kN) |
| 1 | 65 | 33 | HPMC K4M | 7 |
| 2 | 65 | 33 | HPMC K4M | 17 |
| 3 | 33 | 65 | HPMC K4M | 7 |
| 4 | 33 | 65 | HPMC K4M | 17 |
| 5 | 33 | 65 | HPMC K100M | 7 |
| 6 | 33 | 65 | HPMC K100M | 7 |
| 7 | 33 | 65 | Ethocel 7 std. | 17 |
| 8 | 33 | 65 | Ethocel 7 std. | 7 |
| 9 | 33 | 65 | Ethocel 100 FP | 17 |
| * All formulations included 1% of Mg Stearate and 1% of Colloidal Silicon Dioxide | ||||
Table 1: operative and formulative parameters of mini tablets batches.
3. Materials and methods
In this study 9 batches of minitablets (diameter 2 mm, average weight 7 mg) were obtained by direct compression using a rotary tablet press (Prexima 80, IMA) equipped with 2 EU-D punches fitting 24 mini-punches.
As a model soluble drug Theophylline was used combined with different cellulose derivates (hydroxypropylmethylcellulose, HPMC or ethylcellulose, Ethocel) having different molecular weights or particle size (HPMC K4M, HPMC K 100M, Ethocel 7 Standard, Ethocel 100 FP).
4. Results and discussion
The results showed that using the proper operative conditions it was possible to obtain stable process: this could be reflected in low relative standard deviation even on compression (analyzed on upper and lower punch) than on ejection force (Graph 1 and 2).

Graph 1: compression forces evaluation over the batches.
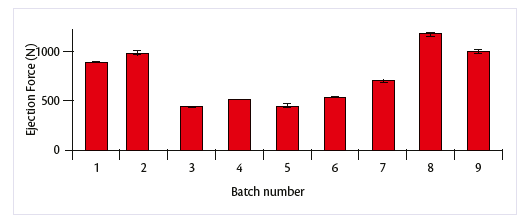
Graph 2: ejction forces evaluation over the batches.
The technology characteristic analysis, resumed in Table 2, showed that all the batches complied with the European Pharmacopoeia for the uniformity of mass and only one batch did not comply with the friability.
| Batch | Unif. of mass | Crushing strength ± SD (N) | Friability (%) |
| 1 | comply | 11.0 ± 1.2 | 0.51 |
| 2 | comply | 20.5 ± 2.5 | 0.23 |
| 3 | comply | 18.9 ± 4.1 | 0.44 |
| 4 | comply | 33.7 ± 3.4 | < 0.01 |
| 5 | comply | < 10 | 1.7 |
| 6 | comply | 23 ± 3.3 | 0.34 |
| 7 | comply | 15.6 ± 3.4 | 0.58 |
| 8 | comply | 27.1 ± 5.26 | 0.58 |
| 9 | comply | 27.5 ± 4.5 | 0.59 |
Table 2: uniformity of mass, crushing strength and friability of minitablets batches.
Regarding the effect of formulative parameters on TH release, data reported in Graph 3 showed that all the matrix minitablets produced had modified release compared to pure TH. In fact pure drug completely dissolved within few minutes (t 50% < 1 min, t 90% = 2 min) according to its great water solubility (release profile not reported). Using HPMC 4KM, the results showed that drug release decreased by increasing the compression force while the polymer amount (33 and 65%) had no significant influence.
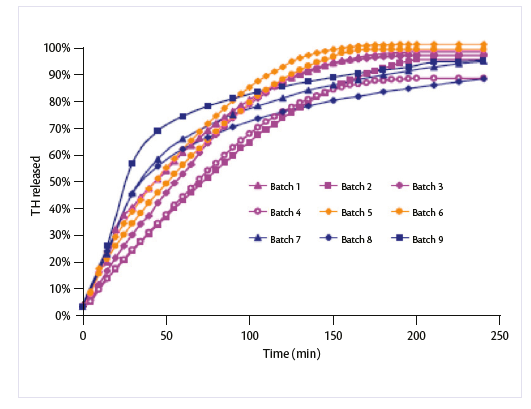
Graph 3: release profile of TH minitablets in pH 7.4 phosphate buffer.
Replacing HPMC 4KM with a HPMC with a higher molecular weight (K100M) the difference in the release profile was observed only at the highest compression force. The release profiles of all matrix minitablets containing Ethocel were observed, as expected, being significantly different from those obtained using HPMC, suggesting a different dissolution mechanism. Moreover it was seen that minitablets prepared using Ethocel at lower molecular weight had slower drug release than minitablets prepared from higher molecular weight grades.
5. Conclusion
Both cellulose derivates allow the production of matrix minitablets containing TH by direct compression. Based on the dissolution profiles, it can be concluded that a more constant and controlled release can be achieved using a hydrophilic polymer (HPMC) releasing the drug by swelling rather than a hydrophobic one controlled by drug diffusion.
References
[1] CC. M. Lopes, J.M. Sousa Lobo, P. Costa, J.F. Pinto, Directly compressed mini matrix tablets containing ibuprofen: preparation and evaluation of sustained release, “Drug. Dev. Ind. Pharm.”, (32) 95-106, 2006.
[2] T. Riis, A. Bauer-Brandl, T. Wagner, H. Kranz, pH-independent drug release of an extremely poorly soluble weakly acidic drug from muliparticulated extended release formulations, “Eur. J. Pharm. Biopharm.”, (65) 78-84, 2007.
Paper Sections
Last Submitted Papers
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
