
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Application of a fractional factorial design for the evaluation of a coating process
1. Purpose

The aim of this work was to gain process knowledge regarding the application of a non functional aqueous coating material on tablets, using a fully perforated pan. For this purpose a 25-1 fractional factorial design was applied in order to screen the critical process parameters, affecting finished product quality and yield.
2. Methods
Round 6 mm, biconvex placebo 100 mg tablets were coated by using a water based material (Opadry II pink at 20% concentration) in a fully perforated pan (Perfima Lab IMA, 30 liters drum, Figure 1). The Experimental Design matrix was executed utilizing two different batch sizes closed to the minimum and maximum capacity of the coating drum (12 L and 28 L). For each batch size 16 experiments plus 3 replicates of the center point were performed (Table 1 and 2). Using prior knowledge of the typical operating parameters of the coating pan, the following five candidate Critical Process Parameters (CPPs) were selected and studied at two levels: Inlet Air Flow Rate (400-700 m³/h), Inlet Air Temperature (65-75°C), Atomization Pressure (1.5-2.5 bar), Spray Flow Rate (40-90 ml/min) and Theoretical Weight Increase (4-7%). Critical Quality Attributes (CQAs) and associated performance indicators were investigated as responses: Tablets Diameter and Thickness Increase, Uniformity of Coating Film and Process Efficiency.
The design was generated and analyzed using Design-Expert® (Stat Ease, MN, US).
| Parameter | Mu | Value |
| Drum capacity | L | 30 |
| Pan speed | rpm | 10 |
| Pan depression | Pa | -30 |
| Type of gun | – | Schlick – S75 |
| Number of guns | – | 2 |
| Nozzles diameter | mm | 1.2 |
| Gun to bed distance | cm | 18/20 |
| Pattern pressure | bar | 2 |
Table 1: fixed parameters for the Experimental Design (both 12 and 28 L batch)
| Batch Size | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
| 12 L | 18 L | A: Inlet Air Temperature | B: Inlet Air Temperature | C: Atomization Pressure | D: Spray Rate | E: Theoretical Weight Gain |
| Std. | m3/h | °C | bar | ml/min | % | |
|
1 A
|
1B
|
400 | 65 | 1.5 | 40 | 7 |
|
2 A
|
2 B
|
700 | 65 | 1.5 | 40 | 4 |
|
3 A
|
3 B
|
400 | 75 | 1.5 | 40 | 4 |
|
4 A
|
4 B
|
700 | 75 | 1.5 | 40 | 7 |
|
5 A
|
5 B
|
400 | 65 | 2.5 | 40 | 4 |
|
6 A
|
6 B
|
700 | 65 | 2.5 | 40 | 7 |
|
7 A
|
7 B
|
400 | 75 | 2.5 | 40 | 7 |
|
8 A
|
8 B
|
700 | 75 | 2.5 | 90 | 4 |
|
9 A
|
9 B
|
400 | 65 | 1.5 | 90 | 4 |
|
10 A
|
10 B
|
700 | 65 | 1.5 | 90 | 7 |
|
11 A
|
11 B
|
400 | 75 | 1.5 | 90 | 7 |
|
12 A
|
12 B
|
700 | 75 | 1.5 | 90 | 4 |
|
13 A
|
13 B
|
400 | 65 | 2.5 | 90 | 7 |
|
14 A
|
14 B
|
700 | 65 | 2.5 | 90 | 4 |
|
15 A
|
15 B
|
400 | 75 | 2.5 | 90 | 4 |
|
16 A
|
16 B
|
700 | 75 | 2.5 | 90 | 7 |
|
CP1A
|
CP1B
|
700 | 65 | 2.5 | 90 | 4 |
|
CP2A
|
CP2B
|
400 | 75 | 2.5 | 90 | 4 |
|
CP3A
|
CP3B
|
700 | 75 | 2.5 | 90 | 7 |
3. Results
Preliminary tests on both batch sizes were performed to investigate factor levels assuring the feasibility of the design. Subsequently the screening design was executed. All experiments resulted in coated tablets free of visible defects and regions with high process efficiency were identified. The ANOVA results showed that adequate mathematical models between the CPPs and CQAs were developed. It was also identified that the various factors, in most of the cases, affected the responses in a different way when the batch size changed. In general, Air Flow Rate and Atomization Pressure were proven as the CPPs predominantly affecting process efficiency and film quality attributes.
The study showed that Process Efficiency was positively influenced by Air Flow Rate and negatively by Atomization Pressure in the 12 L batch while in the 28 L bath Process Efficiency improved when using lower values of Air Flow Rate and Inlet Air Temperature combined with high spraying rates. In addition the several significant interactions, provided the tools for further understanding the complex phenomena governing the coating process, especially at the marginal manufacturing setting expressed by the small batch size compared to the drum’s capacity.
One of the most important findings was that through appropriate factors settings it was feasible to achieve uniform coating for both batch sizes as described by figures from 2 to 5.
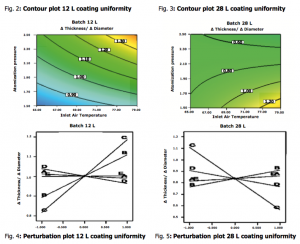
4. Conclusions
Using an economical statistical experimental design within the Quality by Design (QbD) framework a deeper systematic knowledge of the coating process was gained for the formulation studied.
Therefore, the pillars for a sequential process optimization through rationalized factor number reduction and new level selection were established.
References
[1] Cahyadi C., Heng P. W. S., Chan L.W., Optimization of Process Parameters for a Quasi-Continuous Tablet Coating System Using Design of Experiments, AAPS PharmSciTec, Vol. 12, N°1, March 2011, pp. 119-131.
[2] Korakianiti E., Rekkas D., Statistical Thinking and Knowledge Management for Quality-Driven Design and Manufacturing in Pharmaceuticals, Pharm Res 28, 2011, pp. 1465-1479.
[3] Porter S. C., Verseput R. P., Cunningham C. R., Process Optimization Using Design of Experiments, Pharmaceutical Technology, October 1997, pp. 1-7.
[4] Teckoe J., Mascaro T., Farrel T. P., Rajabi-Siahboomi A. R., Process Optimization of a Novel Immediate Release Film Coating System using QbD Principles, AAPS PharmSciTech, Vol. 14, N° 2, 2011, pp. 531-540.
[5] Vesey C. F., Rizzo M., Rajabi-Siahboomi A. R., Identification and Influence of Critical Coating Process Parameters on Drug Release from a Fully Formulated Aqueous Ethylcellulose Dispersion, poster AAPS Annual Meeting, 2007.
