
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Filling and Closing Machines for RTU Syringes, Vials and Cartridges
With the development of new parenteral products and specialized therapies for small patient populations and the increasing trend for user-friendly and safer self-administration systems, pre-filled syringes and, more generally, Ready-To-Use (RTU) components, are becoming the preferred solution among the different parenteral dosage forms.
INJECTA. Fill-finish Solutions for Flexible and Multi-product Manufacturing.
INJECTA. The Queen of Quality
INJECTA's Fully Isolated Robotic Technology Disrupts the Conventional Approach to Aseptic Processing.
INJECTA. Fill-finish Solutions for Flexible and Multi-product Manufacturing.
The growing market trend for pre-filled syringes and other Ready-To-Use components is strictly connected to the high prevalence of chronic diseases across the globe, the increase in the geriatric segment of the population, more sedentary lifestyles, the growing use of safety self-administration devices and the rising demand for vaccines.
On the other hand, the ongoing biopharmaceutical boom (biologic and biosimilar drugs) has also contributed to an increase in products targeted for parenteral delivery, which, in turn, has contributed to promoting the growth of the pre-filled syringe market, as pre-filled syringes are easy to use and ensure a high dosing accuracy while minimizing product loss. Companies targeting biologics face a new frontier in development and manufacturing as these drugs are targeted for smaller patient populations and are difficult to produce owing to the reactivity, viscosity and expense of the macromolecules.
Being patient safety and dosing accuracy the main issues for injectable administration, flexible and multi-product fill-finish operations are becoming more crucial as most of the new injectables require a delivery model that is patient-centric, safe and cost effective. Biologics and biosimilars are more process-intensive and the traditional aseptic process is not well suited to meet the need for medium-to small batch flexibility. Their complexity, as well as their advanced formulation (high-value drugs) change the way products are brought to the market and companies are now looking to reduce the total cost of production, which is higher in relation to pharma drugs.

Today’s challenge for manufacturing companies is to adopt new methods and technologies to process high-value drugs with high-mix batches into multiple formats or sizes (such as vials, cartridges and syringes for parenteral use) so as to ensure a flexible and repeatable process, in a controlled, safe environment. The process must be repeatable and minimize the risks, so as to reduce contamination potential and improve production quality and manufacturing efficiency in an aseptic and sterile fill-finish process. The growing use of a closed robotic filling system is in response to these changes and demands!
Robotic revolution and isolator-barrier systems: the perfect combination for advanced aseptic processing.
Meeting this growing demand for multi-product, fill-finish operations, IMA Life has designed and implemented a flexible and reliable “medium-to-low production batch” scalable solution for the filling and closing of a wide range of Ready-To-Use (RTU) disposable components:
INJECTA, our ultimate, fully robotic fill-finish machine for RTU components pre-arranged in nest & tub or trays.
INJECTA is our innovative approach to deliver a new aseptic filling machine concept for injectable pharmaceuticals. Its Quality By Design (QbD) development process has been outlined to meet the targeted product quality profile and operate in a faster, safer way, so as to proactively respond to the challenges posed by the increasing complexity of new drug substances (medium-to-small batch flexibility). To achieve the best possible improvement in terms of safety and productivity, INJECTA’s full fill-finish process is based entirely on robotics operating in a closed, “towards gloveless”, isolator environment.
In aseptic processing, operators are the primary source of contamination. INJECTA is designed to run with no mouse holes, conveyors, glass-to-glass contact or vibrating bowls which all create risks to the batch. Results: cost and time saving, major safety for operators and, thanks to the isolator environment, an increase in product quality and safety.
Whereas conventional attempts to use robots are typically confined to just one step of production, i.e. simply for moving vials from station to station or for removing the cover from a tub of nested containers, INJECTA uses the full potential of its robots.
Its specialised robots perform all handling activities with no glass-to-glass contact and without operator intervention. INJECTA is designed as a whole, exploiting all robotic capabilities in an integrated system. Kawasaki’s seven-axis robot arms are manufactured for the lowest degree of particle shedding.
They are resistant to positive/negative and high pressures and fully compatible for decontamination using H2O2 vapours. No human intervention is required: all activities/issues are solved through robot interactions.
INJECTA’s specialised robots not only provide precise, consistent handling activities, but also offer a high level of flexibility: they are completely digitally controlled with Industry 4.0 capability, thus allowing a better understanding of the manufacturing processes while meeting quality standards and increasing efficiency (integrated automation platform to drive knowledge/information and promote data-driven decision-making).

INJECTA basis of design is hinges on the following 4 main key- concepts:
Flexibility:
- Multi-product and multi-format handling: INJECTA handles pre-sterilised Ready-To-Use containers (syringes, vials or pre-capped cartridges), pre-oriented vials in trays as well as sterilised vials from the depyrogenation tunnel, allowing high process flexibility and adaptability. No need to change the filling module thanks to rapid switches between different drugs and primary packaging products.
- Robotic Technology: the use of advanced robotically driven manipulations throughout all production operations, from outer/inner bag opening to the stoppering station allows for a very smooth production process, drastically reducing human interventions and cross-contamination risks.

Modularity:
- INJECTA IN-BUILT MODULAR CONFIGURATION (available from 1 up to 10 filling nozzles) guarantees a comprehensive, contained integrated production system with no use of interchangeable production modules to adjust production processes. Injecta is flexible and precise in terms of quality.
Its design is intrinsically modular and meets multiple requirements from small-scale clinical trial batches up to medium-high production levels. - HIGH VERSATILITY IN PRODUCT HANDLING: the RTU CONTAINERS can be accurately and efficiently filled and closed INSIDE A NEST OR BY DE-NESTING OPERATIONS. The robotised individual component handling solution (i.e. de-nesting configuration) is ideal for high-level quality control requirements.
- HIGHEST ADAPTABILITY FOR MULTIPLE LAY OUT SOLUTION AND SHAPES: INJECTA is fit for multiple lay out solutions/requirements: IN-LINE or T-SHAPE LYO configurations, ALL-IN-ONE INTEGRATED FILL-FINISH SOLUTION with combined lyo process and capping operations, dedicated solutions to process RTU vials FROM BULK OR upside down from TRAYS. INJECTA’s production modules (primary package handling, filling, stoppering, loading&unloading and capping modules) can be easily fitted and assembled in various configurations (not only the horizontal standards).
Enhanced quality:
- INNOVATIVE BAG AND TUB OPENING: To meet the regulatory requirements, the whole tub opening unit is completely robotized and under containment. After cutting the bag overwrapped around the tub, the primary package is instantly transferred by an internal transfer port to the isolated, processing area. To preserve the sterility of the tub, the bag flaps are opened by the suction cups, only when the tub is physically transferred to the isolated area.
- GLOVELESS READY: The production flow of the fill-finish process is completely gloveless, so as to improve protection and minimize cross-contamination risks. INJECTA is perfectly integrated with IMA Life’s isolator with reduced shell width. The presence of gloves is minimized around the filling & stoppering “isles”.
- ADVANCED COMPLIANCE: The stoppering process is performed using a linear stopper feeding system. Unlike conventional systems, this innovative group is made of small-sized components ideal for RTP transfer, in compliance with Annex 1 requirements in terms of sterilisation process. An innovative fully robotized system for stopper unit collection & assembly is available as well.
- 100% IN-LINE PROCESS CONTROL: INJECTA is naturally born to be flexible. It can easily combine and perform a fully robotized 100% IPC both while in-nest and in de-nesting configurations. As a de-nesting solution, INJECTA’s flexible handling units allow for precise removal of the components from the nest. As a result, individual components are filled, check-weighed, stoppered and then replaced into the nest, in case of syringes and cartridges, or transferred by a conveyor belt to complete the process
Business drivers:
- SINGLE REJECTION FROM THE NEST OR WITH DE-NESTING: All processed RTU components are individually check-weighed (in case of de-nesting) or check-weighed all together while in nest, with single rejection of each individual non-conformity, safeguarding the nest.
- SCALABLE DESIGN: Unique production platform (from one up to ten filling pumps) performing the same identical product handling, filling and stoppering operations avoiding any additional production process re-qualification with consequent time-to-market reduction.
In conclusion, the intensive use of advanced robotically driven manipulations versus conventional handling systems saves process time and costs, thus improving product quality and manufacturing efficiency.
The result is “less time” spent validating aseptic conditions and superior agility for multi-product manufacturing: no size part changeover, rise of process capacity, minimization of product losses, reduced human intervention, smaller footprint. Pharmaceutical companies that move products to market faster will beat their competitors in an era that demands greater agility.
INJECTA. The Queen of Quality
The pharmaceutical industry has been increasingly embracing injectables as a consistently profitable business sector.
For decades, injection has been largely limited to in-patient use, with health professionals actively involved in administering doses intravenously, intramuscularly and subcutaneously. The introduction of prefilled syringes and injection devices has reduced the fear factor associated with outpatient injections. In short, injection has evolved from a being a last choice dosing option for patients.
These syringe and cartridge based packages also reduce the possibility of errors in preparing and administering a subcutaneous injection.
However, it may not be surprising to find that all of the top 10 pharmaceuticals by sales in 2018 were injectable, most of which are biologics and biosimilar: the future expansion of injectables is directly linked to the future of biologics.
The industry shift to biologics has established a growing market for injectables and supporting technologies. The pharmaceutical industry is adopting more and more emerging technologies to improve product quality and manufacturing efficiency. Considering the steady growth of these next-generation therapies, Pharma companies’ main concern focuses on product quality rather than production performance. We can therefore claim that flexibility in manufacturing is fundamental to production success. Flexible facilities allow production of different product volumes that can quickly respond to changes in expected market demand and enable rapid switching between different products and product packaging (i.e. vials, cartridges or syringes for parenteral).
Pharmaceutical companies that move products to market faster will beat their competitors in an era that demands greater agility.
 Within this scenario, we have come up with INJECTA, a new concept of aseptic filling machine for injectable pharmaceuticals. Our aim was to adopt an innovative approach to handling nested syringes, vials and pre-capped cartridges. We were motivated by the notion that conventional, fill-finish lines are not flexible in terms of primary packaging materials (such as vials, syringes and cartridges) as expected by the latest market requirements. They fail to meet the current need to produce a varied product portfolio. When therapies require smaller volumes and there is a higher number of different products to manufacture in medium to low production batches, flexible methods of production are essential.
Within this scenario, we have come up with INJECTA, a new concept of aseptic filling machine for injectable pharmaceuticals. Our aim was to adopt an innovative approach to handling nested syringes, vials and pre-capped cartridges. We were motivated by the notion that conventional, fill-finish lines are not flexible in terms of primary packaging materials (such as vials, syringes and cartridges) as expected by the latest market requirements. They fail to meet the current need to produce a varied product portfolio. When therapies require smaller volumes and there is a higher number of different products to manufacture in medium to low production batches, flexible methods of production are essential.
INJECTA responds to this demand for high flexibility and to the challenges of the ever-increasing complexity of new drug substances. The use of advanced robotic driven manipulations versus conventional handling systems actually improves product quality and manufacturing efficiency.
INJECTA can handle pre-sterilised Ready-To-Use containers (syringes, vials or pre-capped cartridges), pre-oriented vials in trays as well as sterilised vials from depyrogenation tunnel, permitting high process flexibility and adaptability.
Specialised robots perform all handling activities with no glass-to-glass contact and in the absence of operator intervention. The result is “less time” spent validating aseptic conditions, and superior agility for multi-product manufacturing. A very high level of modularity means INJECTA can be set for 1 or 2 filling groups for clinical trials of small production badges and for 5 or 10 filling groups for high production demand.
It can be equipped with peristaltic or volumetric dosing pumps which are driven by the same system.
Winning features:
- Revolutionary in-line 100% process control, with single component reject.
- Robot asynchronous movements, allowing single operation completion.
- Empty tub/tray transfer performed at robot base level without the use of conveyors.
- Use of a linear stopper feeding system, avoiding particle generation from vibratory bowls.
- De-nesting operation available for high-level quality control, with robot individual component handling.
The ultimate developments provides further benefits:
- All the operating units (filling, IPC & stoppering) are located in a remote location from the product path.
- Further risk deduction is achieved by integrating the fill-finish process within gloveless barrier isolation systems.
- Easier access to filling and stoppering areas from both sides of the machine.
- Improved protection of the fill-finish process by an uninterrupted unidirectional airflow.
- Advanced ergonomics with air recirculation duct redesign.
- Reduced isolator shell width with positive impacts on space ergonomics.
By embracing and adopting new robotic technologies throughout all production operations, from outer/inner bag opening to the stoppering station, INJECTA allows for a very smooth production process, drastically reducing human interventions and therefore cross-contamination risks.
INJECTA can be further integrated with an in-line lyophilisation process, where an automated loading/unloading system handles products into and out of the freeze dryer. At the end of the lyophilisation process, the pressure applied by the freeze dryer shelves closes the vials. Then, stoppered vials move to capping operations, relying on conventional standard primary packing components (alu caps).
INJECTA’s fully automated technologies fulfil Authority guidelines for data integrity and keep up with new industrial principles.
In line with Industry 4.0 requirements for interconnected production data, INJECTA’s integrated automation platform allows for complete data accessibility and circulation, efficiency and ability to exchange big volumes of data.
In short, considering that pharmaceutical companies need technology support for automated and interconnected manufacturing, we can say that INJECTA, by providing improved quality and flexibility in the production process, can fully achieve this reality. When effectively implemented, automation can increase efficiency, productivity and quality while reducing costs and time-to-market.

INJECTA's Fully Isolated Robotic Technology Disrupts the Conventional Approach to Aseptic Processing.
Central goal is to achieve flexible production in a safe, aseptic environment, implementing cost-saving solutions with a space-efficient design. In aseptic processing, operators are the primary source of contamination. Although human operators figure in a potentially risky activity in sterile areas, pharma manufacturing remains predominantly human. Therefore, one of the focal topics for pharmaceutical manufacturing today is how to improve the use of robotic automation in a gloveless, isolated environment. The ideal outcome is the creation of a digitally controlled aseptic process with little to no human intervention.
To address this issue, in many pharmaceutical industries, conventional filling systems are now progressively implemented with robots. However, these robots are typically used for only one step of production, i.e. for simple moving of vials from station-to-station or for removing the cover from a tub of nested containers. However, these attempts generally represent only half-steps towards utilizing the full potential of robots.
To achieve the aim of reaching the maximum possible improvement in safety and productivity the full fill/finish process should be completely based on robotics operating in a closed, towards gloveless, isolator environment.
INJECTA can meet these fundamentals, tracing the roadmap for a real and complete fill-finish process modernization. Its fully isolated robotic filling system is designed as a whole, an integrated system utilizing all robotic capabilities. Kawasaki’s seven-axis robot arms are made in chrome aluminium and designed for the lowest particle shedding. They are resistant to positive/negative and high pressures and fully compatible for decontamination using H2O2 vapors.
INJECTA’s specialized robots not only provide precise, consistent handling activities, but also offer a high level of flexibility: they are completely digitally controlled with Industry 4.0 capability. Minimum human interventions are required: major activities/issues are solved through robot interactions.
If flexibility is crucial for high-value and medium-low production batches, INJECTA is naturally born to be flexible. Its filling system can accommodate a wider range of containers (tub, nest and trays) and components (vials, syringes and cartridges) with minimal or no size changeover. Further to this, its flexible methods of production allow both the bulk processing of vials and the processing of Ready-To-Use components.
It can easily combine robotized in-nest or de-nesting operations before filling or after stoppering.
INJECTA guarantees a whole, contained integrated production system with no use of interchangeable production modules to adjust production processes. INJECTA is flexible and precise in terms of quality. It works with multiple primary packaging components. Its design is intrinsically modular and meets multiple requirements from small-scale clinical trial batches up to medium-high production levels. INJECTA supports a completely automated fill/finish process without the lowest presence of gloves and glove ports, guaranteeing a greater level of sterility. Single-use materials such as Ready-To-Use primary containers, disposable inner/outer bags, etc. are used and disposed of within the system.
We can definitively state that the robotic technology is a real new challenge in terms of greater accuracy, innate modularity, flexibility, and reliability. The risk for microbial contamination generated by operating personnel is virtually eradicated by its advanced aseptic production system. Towards gloveless solutions drastically remove the potential for human contamination and guarantee a greater level of sterility.
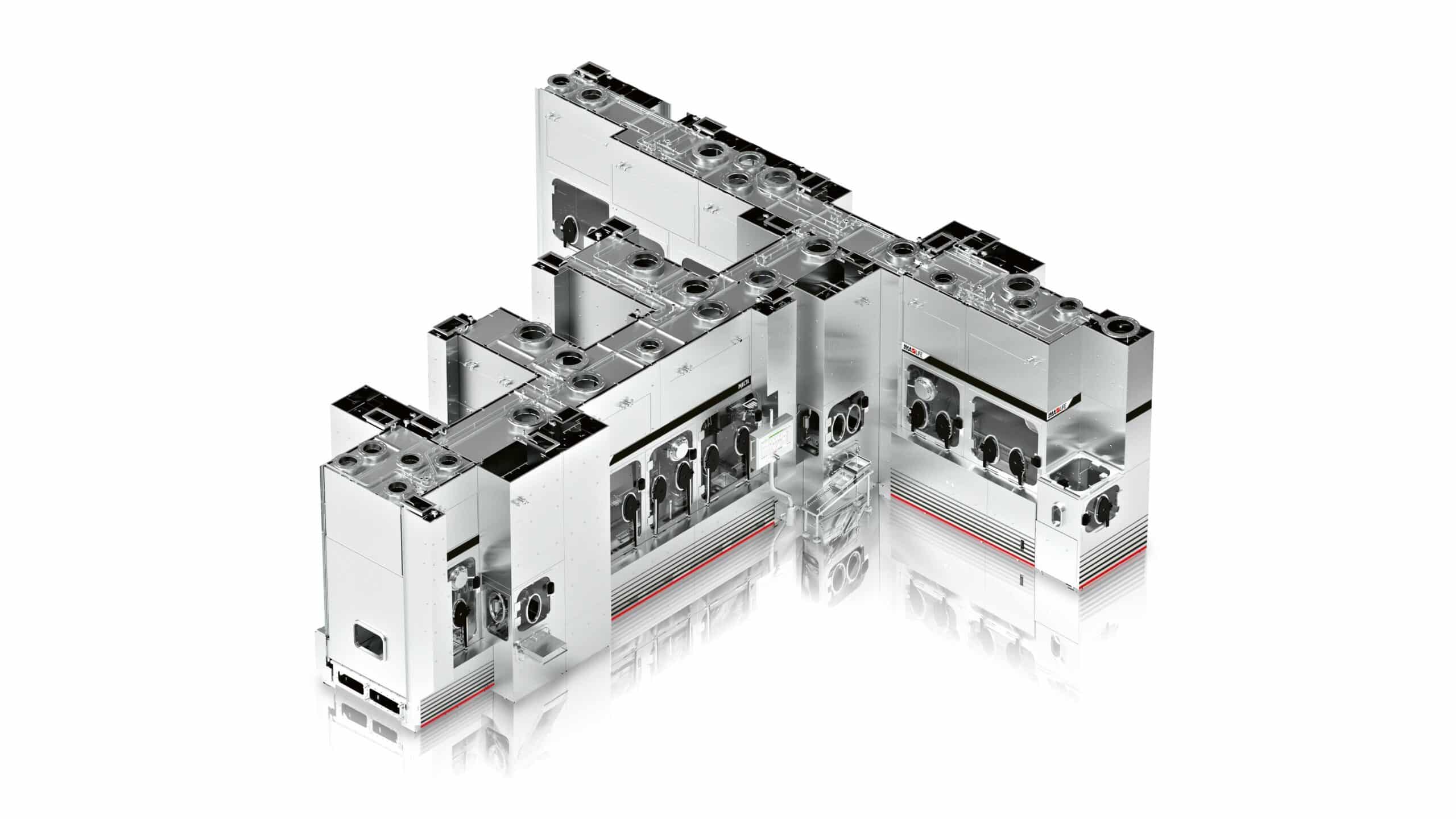
 Fig.1: Advanced robotic handing
Fig.1: Advanced robotic handing

Fig.2: Robot overview

Fig.3: Pre-sterilised Ready-To-Use components pre-arranged in nest & tub and trays
Matching with IMA isolator system
INJECTA is an extremely flexible machine that allows switching between production cycles of different pharmaceutical forms (syringes, vials or pre-capped cartridges) enabling the replacement of machine parts more quickly than a standard machine.
This engineering solution leads to considerable advantages in isolated lines because, based on the line layouts and on the needs of each individual customer, it allows the isolator to be designed in a more ergonomic way having to interface with a single machine.
Design optimisation: mock-up
IMA Life – as a global supplier of complete lines of isolators – can make the most of INJECTA’s flexibility to create the layout most suited to each customer needs and check its ergonomics and design during specific design review phases. Through a wooden mock-up of the complete filling line and the related isolator, made to 1:1 scale, all the activities to be performed on the line are tested (e.g. assembly of disposable systems, etc.) and so is the interaction between INJECTA and the other units upstream and downstream of the line, also made of wood and full scale. As the mock-up of INJECTA is implemented, the positioning of the utilities inside the isolator (e.g. water, nitrogen, compressed air and steam) is also defined which, thanks to the single block design, do not need to be connected/disconnected during production. The positions of the utilities will therefore be set so as not to affect the ergonomics of the line.
WIP (Washing In Place) cycle
The optimised design of the isolator on a single machine as well as the use of easily cleanable robots capable of moving independently also allows the WIP (Washing In Place) cycle to be optimised. Consequently, considering the worst case filling scenario, it will be enough to validate a single washing cycle.
VPHP biodecontamination cycle
During the filling line setting-up phase, by analysing specific customer production requirements (i.e. multiple filling processes and production parallelism), it is possible to develop a single decontamination cycle with VPHP. The specific set-points for the decontamination process, once validated, do not require modification or re-testing since it is a dedicated cycle for a single line (INJECTA + isolator).
Developing and validating a single VPHP cycle, for all the possible configurations of INJECTA, results in time saving both for the first validation and for periodic re-validations.

Fig.4: Example of mock-up
Leak test
The installation of INJECTA is done directly inside the isolator in the configuration defined as “6 walls” where the sealing of the parts is guaranteed by the presence of fixed seals around the machine components.
The integrity of the seals and all the other components present in the isolator (e.g. bulk heads, doors, RTP, gloves, drains, piping system) are guaranteed by performing a leak test before each VHP cycle. Since all the components are fixed, verification will be done on a single leak test – increasing its success probability.
The installation of a single assembly (INJECTA +isolator) makes it possible to define the leak test parameters of the entire line in a single action – without any need for re-testing.
Software and hardware interaction
Also in terms of electronic and electrical interaction, communication between INJECTA and the isolator does not require any changes in the transition from one filling configuration to another, since disconnecting and reconnecting is not necessary in the size changeover phase. The electronic and electrical connections, just like for the utilities, are defined in the design phase of the machine and tested during the line mock-up phase.

Fig. 5: Washing in place
Environment classification
The environmental rating of INJECTA is guaranteed by defining specific air speed, temperature and humidity set points reached immediately after the installation of the line to comply with current regulatory requirements. Thanks to the presence of fixed and combining modules, these parameters are defined as early as during the preparation of the quotation and the definition of the line lay-out, through the analysis of the customer’s URS. A single balancing of the parameters is therefore necessary, without any need for further checks during the production process.
All this leads to time saving for the first and subsequent validations of the isolator, as well as shorter waiting times between one production batch and another.
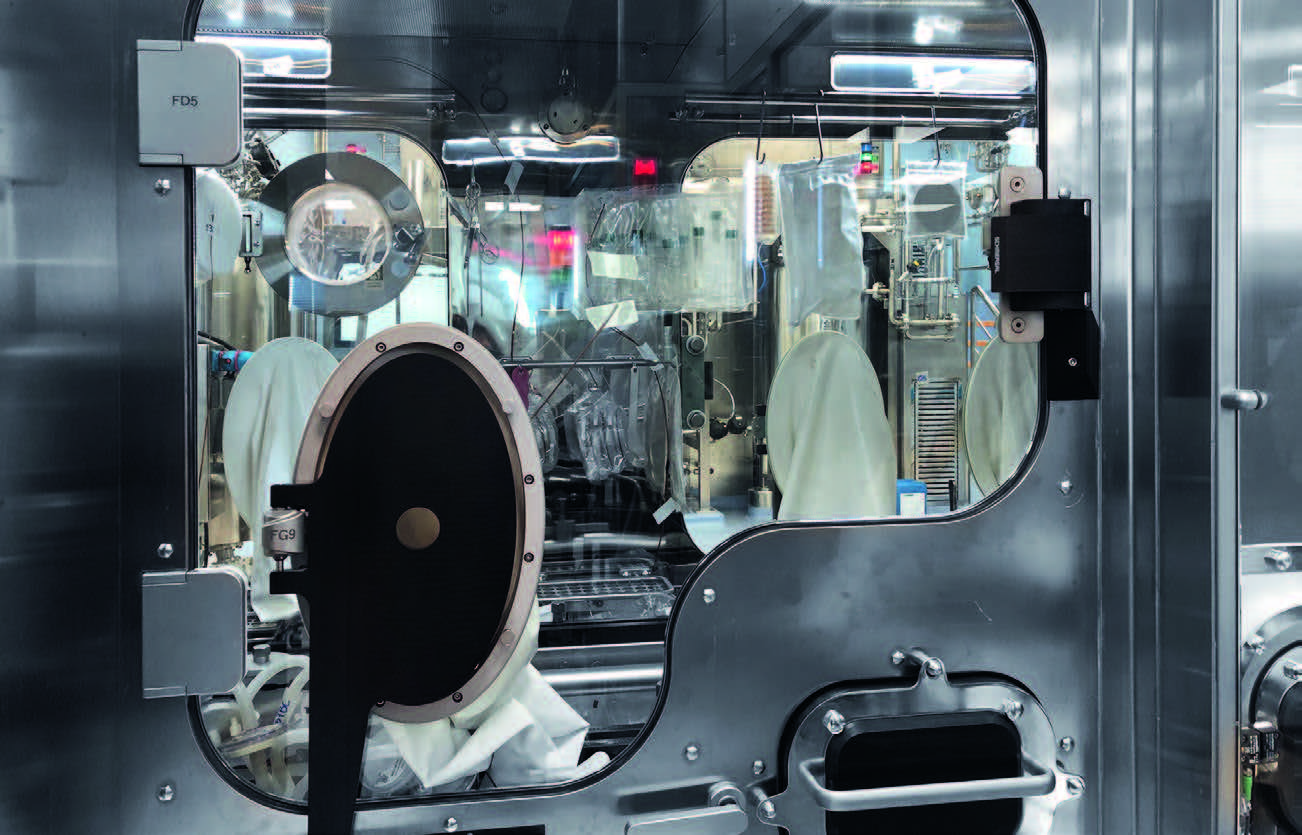
Fig.6: Environmental Classification

Fig.7: Example of a double wall isolation solution.
Environment condition
Inside the isolator it is possible to set the T/RH% and air speed parameters thanks to the air treatment unit (HVAC) which creates different environmental conditions according to production needs. In the absence of an HVAC unit, the environmental conditions inside the isolator are defined by the surrounding installation environment, with direct air intake from the classified room.
INJECTA isolated robotic filling system intrinsically entails all these benefits. It represents our approach to INDUSTRY 4.0 requirements and reinforces our vision: to create value for customers by means of advanced technology, process improvement and service support.
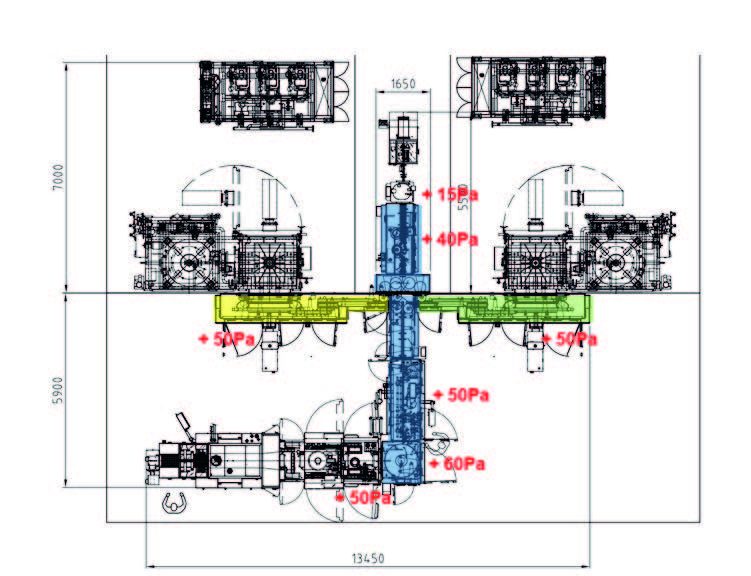
Fig.8: example of pressure cascade
