
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Aseptic Processing
The main concern of the pharmaceutical packaging market has always been the integrity of the drug, and, in the case of parenteral dosage forms, its sterility.
Vaccine manufacturing lines from a single source
Vaccine manufacturing lines from a single source
The universal impact of the disease, the uncertain circumstances and the urgent search for effective vaccines constitute a significant challenge for the pharmaceutical sector.
There is a growing need to supply a global market with innovative medicines at a profitable cost. So pharmaceutical equipment manufacturers have to react quickly, while maintaining due levels of diligence and social responsibility.
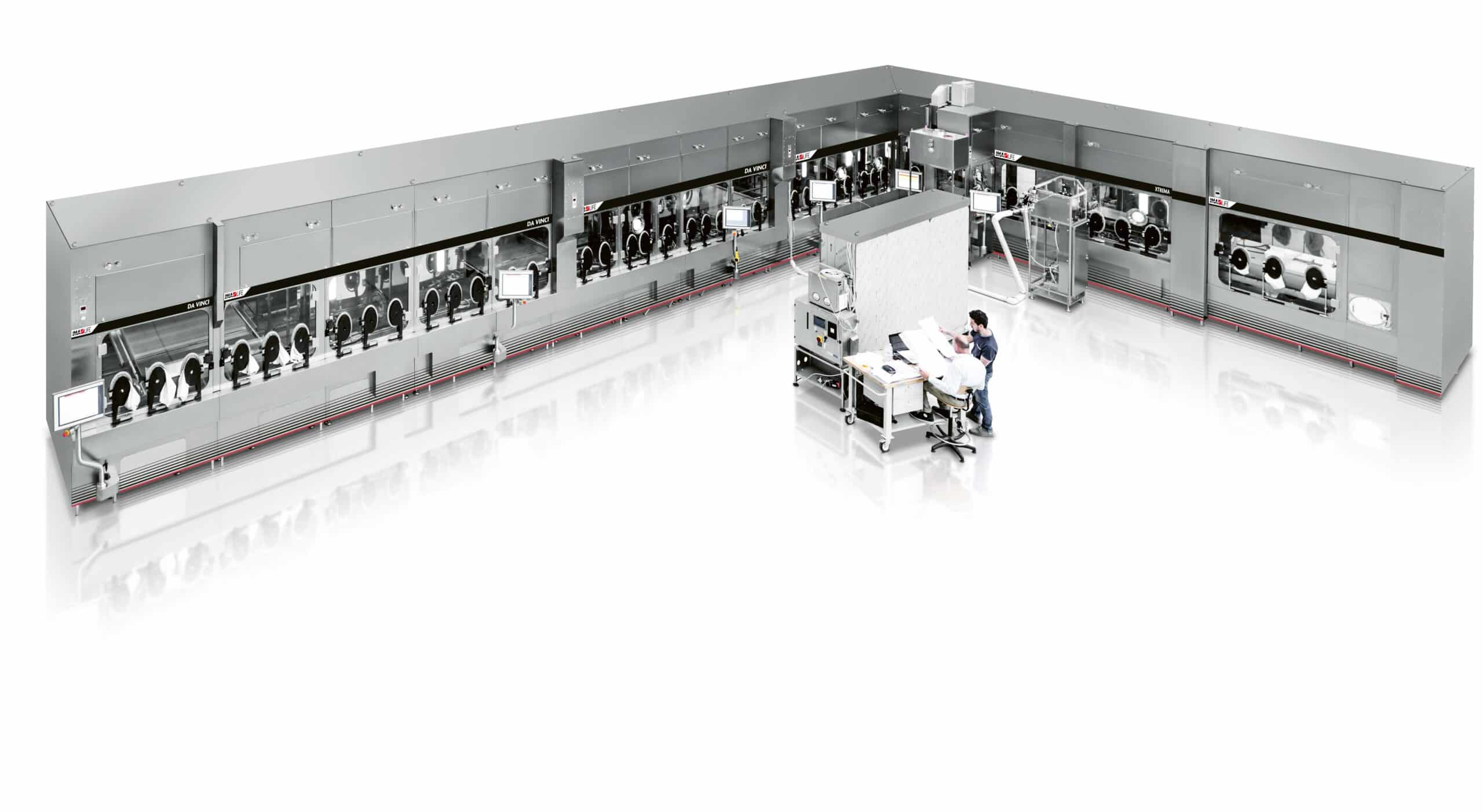
To meet this demand, we at IMA Group operate in highly specialised pharmaceutical divisions, focusing on specific processing and packaging areas, in order to provide our customers real added value based on two main criteria. Firstly, the ability to design and supply equipment and advanced technology that is versatile and can be adapted for new products. Secondly, insider knowledge of manufacturing processes and equipment applications, which enables us to actively collaborate with the end user in the optimisation of processes and the joint development of new ideas. The design of innovative, safe, automated high-speed lines for the processing of injectable products (such as vaccines, oncology drugs and so on) requires great flexibility in order to offer highly customised solutions. Thanks to its extensive portfolio, IMA can support the pharmaceutical industry with complete high-speed lines for vaccines including primary and secondary packaging solutions.
Primary packaging for vaccines
IMA Life (Aseptic Processing & Freeze-Drying Solutions) is one of the three pharmaceutical divisions of IMA Group, world leader in the design and manufacture of complete aseptic lines for parenteral products from the initial washing of the bottles, through depyrogenation, filling, capping, and decontamination, up to and including secondary packaging. All this with or without freeze-drying systems and, in the section where the product is exposed, the use of an isolator or other containment solutions.
IMA LIFE aseptic filling lines are intended for applications requiring the most scientifically and technologically advanced equipment, where the industry is obliged to comply with very strict standards (GMP – Good Manufacturing Practice). Since vaccines come in either liquid or lyophilized powder form, our comprehensive product range means that IMA Life can process both liquid and lyo products.
At IMA Life, we have learned to design solutions alongside our customers, providing tailored responses to each and every request from the aseptic, biopharmaceutical and pharmaceutical markets.
Our aseptic high-speed processing lines feature cutting-edge robotics and fully integrated isolation technologies, ensuring line performance of 400 to 600 units per minute. Designed around a modular concept, they ensure great flexibility, production efficiency and product quality, which can be achieved in the safest way possible and to tighter schedules. Perfect for the production of vaccines.
Six different production sites located in Italy, the USA and China, each one specialised in a specific step of the process, ensure a technologically advanced product portfolio. Each solution makes use of consolidated technology and is the result of not only more than 60 years’ experience, but also ongoing partnerships and large-scale projects, developed and managed with leading international pharmaceutical companies.
Secondary packaging for vaccines
IMA Safe is the IMA Group division that specialises in secondary packaging with deep-draw thermoformers, paper tray packaging machines, horizontal and vertical cartoners, as well as complete end-of-line solutions made by BFB.
Cartoners are designed to run efficiently, reliably and with fast changeover times, ensuring easy synchronisation with both upstream and downstream machines, thus establishing the link between primary and secondary packaging.
 Both machines and feeding units are extremely flexible, enabling several types of products and packs to be processed. They can handle a wide range of very delicate and fragile products such as ampoules, vials, syringes and many others, thanks to the use of robotic systems applied to the most critical areas like product feeding and connection from the upstream machine to the cartoner.
Both machines and feeding units are extremely flexible, enabling several types of products and packs to be processed. They can handle a wide range of very delicate and fragile products such as ampoules, vials, syringes and many others, thanks to the use of robotic systems applied to the most critical areas like product feeding and connection from the upstream machine to the cartoner.
IMA Safe is able to supply the most advanced solutions for handling vaccines, not only from single to multiple vial feeders inside a tray, but also multi-product trays. Constant research and innovation have made it possible to develop optional devices and other ancillary equipment applications such pre-filled syringes, swabs, needles or cannula feeders, to name just a few, and to supply customised packaging solutions. Sustainability in packaging is a topical issue, and in response to increasing concerns about packaging waste, manufacturers have made significant efforts to improve in this area.
IMA Safe has developed smart solutions to package parenteral and very fragile products such as vials, ampoules and syringes on paper trays inside a carton to create 100% paper-based packaging, reducing the environmental impact while ensuring, a high level of product protection.
IMA Safe proudly supports more environmentally friendly vaccine packaging.
Not only does packaging have the functional purpose of protecting, conserving and transporting the product, it also has an informative purpose and very often contains data unseen by the end consumer.
This data is essential to protect against counterfeiting and to facilitate the tracking of
the product at arrival and distribution points.
At IMA BFB division, we design and manufacture end-of-line equipment and we can count on the most comprehensive range of secondary packaging machines in order to protect the product and the information within. Flexibility, customised solutions
and engineering capabilities, together with a highly professional service network, are the ingredients which help our customers gain a competitive advantage. Our wide range of overwrapping, banding and hrink-wrapping machines can process any type of pack guaranteeing high efficiency even with unstable products. Whichever type of case packing you are looking for, we can supply it. From wrap-around to side or top loading, all our models are conceived with reduced footprints, while assuring ergonomics and easy operator access for any type of cleaning or maintenance operations. All of our solutions are devised to preserve the integrity of the contents, ensure total control throughout all stages of the serialization process and enable safe storage.
Our range includes integrated case-packing and palletizing solutions and a robotized de-palletization system for different products, such as jars, vials and bottles positioned in heat formed trays. We have recently developed innovative new product handling solutions for blow-fill-seal containers including those designed for inhalers, pens or auto-injectors and other highly sensitive pharmaceutical components.
It is our mission to devise optimal turnkey solutions for different applications with the right combination of high production speeds.
