
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Formulation and Lyophilization Process Development for Endotoxin Kits.
Introduction
Parenteral drugs or products are sterile preparations containing active pharmaceutical ingredients and excipients intended to be administered by injection, infusion, implantation or by any route aside from the alimentary canal.1 These drugs are typically packaged in either single-dose or multidose containers and their testing for pyrogens is critical to patient and animal safety. While a variety of microorganisms like bacteria, molds, yeasts and viruses can produce pyrogens, due to its high potency and ubiquitous nature, bacterial endotoxin is the only significant pyrogen of concern to the parenteral drug industry.2,3
Bacterial endotoxins are found in the outer membrane of gram-negative bacteria which are members of a class of phospholipids called lipopolysaccharides (LPS). LPS are not exogenous products of gram-negative bacteria. The release of LPS from bacteria like Escherichia coli, Proteus, Pseudomonas, Enterobacter and Klebsiella takes place after their death and lysis and is refractory to removal via sterilizing membrane filters.4 Therefore, it is possible to have a sterile solution that has sufficient endotoxin to be pyrogenic. The only practical way to avoid endotoxin contamination is to eliminate it at the outset by using endotoxin-free materials and aseptic techniques throughout the parenteral drug manufacturing process.
The first bacterial endotoxin test [BET] for pyrogen detection was an expensive, time-consuming rabbit fever assay. The rabbit test was replaced by an in vitro test in the 1960s that utilized Limulus amebocyte lysate (LAL).5 LAL is now the ‘minimum standard’ for endotoxin testing and standard procedures must reference the USP <85> Bacterial Endotoxins Test.6 While LAL is a relatively simple test, the preparation of its controls can be tedious, and the assay demands attention to detail to ensure an acceptable accuracy and reproducibility. Most importantly, the lysate for LAL test is sourced from the blood equivalent of horseshoe crabs, which are limited in supply and, therefore, not sustainable in the long term.7, 8
To overcome the challenges associated with an LAL test, recombinant Factor (rF) based-BET assays have recently emerged as an ‘animal-free’ alternative to LAL assays. These rF assays have been successfully validated and possess specificity and sensitivity advantages over the LAL assay.9 Additionally, due to their animal-free origins, rF based-BET assays enable a sustainable supply as well as a scientific characterization of lysate which was not possible for the legacy LAL.10 Finally, it offers a ‘future proof’ path to implement a 3Rs (Replace, Reduce and Refine) concept which is being increasingly incorporated into legislation, guidelines and practice.11
BET Market Forecast
The global endotoxin market is expected to increase from 767.1 million USD in 2020 to 1.84 billion USD by 2028 at a Compound Annual Growth Rate (CAGR) between 8.5 % – 11.5 %.12, 13 Besides an increase in the market share of parenteral products, an increase in growth in the medical device industry, packaging and raw material production sector is fueling the overall growth of the global BET market.
Lyophilization of Recombinant Factor-BET Assay
Currently, LAL assay dominates the global BET market, but this trend is rapidly changing due to
the advantages offered by recombinant proteins discussed earlier. To improve the shelf storage life
of recombinant factor-based BET kits, several key players in the BET market have approached the IMA Process Development Lab located in Buffalo, NY, for exploring lyophilization of the rF based-BET kit reagents. However, the science required for developing recombinant factor-based BET kits with lyophilized reagents is still at infancy as observed by the lack of lyophilized rF based-BET kits in the global market.

Figure 1. Predicted CAGR of the global BET market
To solve the challenge of improving the stability of rF-formulations, the IMA Lab4Life lyo lab. embarked on in-house projects to shortlist excipients that can improve post lyo-storage stability of the formulation. Key requirements included the acceptable stability of the rF protein during drying stages of the lyophilization process, generation of a pharmaceutically elegant cake and the use of elevated drying temperatures to confine the overall cycle time to < 80 hours.
Thermal Characterization of Prelyo-Formulation
With the industry-leading formulation and lyo-process expertise, the IMA Lab4Life lyo lab. embarked on a mission to identify a fit-for-purpose formulation and process development approach by utilizing an in-house Differential Scanning Calorimeter (DSC) and Freeze-Drying Microscope (FDM). It is critical to note that the pre-existing concentration of sodium chloride in as-provided rF-formulation was ‘locked’ because it was essential for the optimum biochemical activity of the rF protein and hence could not be altered. This led to prelyo-formulation challenges like very-low Tg’ and downstream effects like reduced mannitol crystallization, in line with the published literature.14 Therefore, IMA Lab4Life lyo lab. implemented a scientifically designed formulation optimization scheme and analyzed the impact of several modifications via DSC and FDM as shown in Table 1. This resulted in identification of a unique mannitol-sucrose-NaCl coformulation which offered a desirable fusion of lyoprotection and process aggression capability. The experimental results were in agreement with published literature.15
| Analyte | Tc [°C] | Tc [°C] | Tg” [°C] |
| rF-formulation | -44.5 | ~ -55 | ~ -35 |
| 4:1 M:S 15% in rF-formulation | -40.3 | -43.8 | — |
| 3:1 M:S 10% in rF-formulation | -40.9 | -43.8 | — |
| 3:1 M:S 15% in rF-formulation | -40.0 | -43.5 | — |
M:S – Mannitol-Sucrose
Table 1. Glass transition (DSC) and collapse temperature (FDM) data leading to selection of the rF-formulation with 3:1 Mannitol: Sucrose ratio.
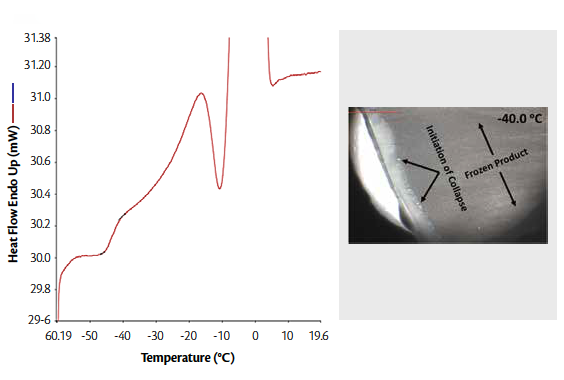
Figure 2 (left). DSC thermogram of rF-formulation with 3:1 Mannitol: Sucrose showing thermal transitions
Figure 2 (right). Collapse temperature of the selected rF-formulation with 3:1 Mannitol: Sucrose
Process Development of the IMA Optimized Formulation
After identification of the optimized candidate formulation, two lyophilization cycles were executed in the IMA-Lyofast 3 (2.3 m2) development lyophilizer to assess the efficacy of the selected formulation. The initial cycle (C1) was executed at a low-pressure-low temperature process setting, while the second cycle (C2) was executed at a higher-pressure-higher temperature process setting. The process plot for C2 is shown in Figure 3.
III.c. Quality Attribute Analyses
The pharmaceutical elegance of the lyophilized cake obtained from C2 was good as seen in Figure 4. The cake structure was uniform with little evidence of microcollapse due to the presence of sucrose.
It is important to note that it is well-established in the industry that a partial microcollapse typically has no deleterious effect on the protein activity and storage stability.16 The residual moisture of the lyophilized cakes obtained from C2 was < 1 % (n=3) indicating a successful yophilization process. The reconstitution time of the lyophilized cake obtained from C2 was acceptable (< 1 minute) and a high temperature [HT-DSC] scan of lyophilized cakes from C1 (red line in Figure 5) revealed the presence of mannitol hydrate in the cake, which are undesirable for storage stability due to their potential to release the bound water with the passage of time. To eliminate the presence of mannitol hydrate, an annealing step was introduced in C2 to increase mannitol crystallization and the secondary drying temperature setpoint was increased to 40 °C as per reported literature.14, 15 The HT-DSC scans of product cakes from C2 (blue line in Figure 5) confirm the absence of mannitol hydrate in the lyophilized cake. Due to absence of mannitol hydrate in cake obtained from C2, the sample cakes were pooled for the accelerated storage stability program at 40 °C and a higher temperature setpoint.

Figure 3. The lyo-process plot of cycle 2 (C2) showing the product temperature profile and utilization of Pirani: Capacitance Manometer comparati-ve pressure ratio for determination of the end of the primary drying.

Figure 4. The appearance of formulation cake from the center and edge vial locations of the C2 cycle executed in the IMA-Lyofast 3 (2.3 m2) development lyophilizer
Summary and Conclusions
The activity of rF protein in the product cake at time (t0) is excellent and results observed after t1 (1 month) indicated a slight but acceptable reduction in the activity of the rF-protein. The extrapolated results from the initial stability timepoints indicate that lyophilized product may be stable for > 1 year at 25 °C or below, thereby suggesting a high probability of success of the lyo-development program with the coformulation approach. Overall, this challenging and time-sensitive project highlights the strengths and capabilities of the IMA process development lab for its global customer base. As is often true, the formulations received by IMA Lab4Life lyo lab. are locked with little or no room for further optimization. However, the lyo experience accumulated by the IMA Lab4Life lyo lab. over the last decade allows its drying process development team to innovate and implement formulation and lyo-process solutions in line with industry best practices. From an industry standpoint, it is evident that the current solution implemented by the IMA Lab4Life lyo lab. will play a critical role in the implementation of 3Rs as an essential 21st century framework for sterility validation industry. At the end of the day, where will the BET industry migrate when the final horseshoe crab is bled, and we run out of LAL supply?

HT-DSC scan of the lyophilized cakes obtained from Cycle 1 (C1) and Cycle 2 (C2). The absence of mannitol hydrate is evident in C2 (blue trend).
References Webster Dictionary
[1] Cooper JF. Bacterial endotoxins test. In: Prince R, ed. Microbiology in Pharmaceutical Manufacturing, 2nd Edition. Bethesda, MD, Par. Drug Assoc., 2008; Vol 2:245-274. 7. [1] Cooper JF, Williams K. Pyrogenicity Case Studies. In: Williams K, ed. Endotoxins, 3rd Edition, New York: Maracel Dekker, 2007:532-540.
[1] Bacterial Endotoxins/Pyrogens | FDA]. Accessed: October 2021.
[1] Williams K. Endotoxins. 3rd Edition. Informa Healthcare, New York. 2007:27-90.
[1] Bacterial Endotoxins | USP. Accessed: October 2021.
[1] Changing+Global+Perspectives+on+Horsesho (horseshoecrab.org), Accessed: October 2021
[1] The Consumer Role in Conservation (horseshoecrab.org) Accessed: October 2021
[1] Piehler et. al., Comparison of LAL and rFC Assays—Participation in a Proficiency Test Program between 2014 and 2019. Microorganisms 2020, 8(3), 418.
[1] Recombinant Factor C assay to aid demand for LAL endotoxin testing (cleanroomtechnology.com). Accessed: October 2021.
[1] 3Rs: Sustainability in Endotoxin Testing | Charles River (criver.com). Accessed: October 2021.
[1] Endotoxin Testing Market Expected to Grow at 8.5% by 2029 (acutemarketreports.com). Accessed: October 2021.
[1] Endotoxin Testing Market To Reach USD 1,843.4 Bn By 2028 (reportsanddata.com). Accessed: October 2021.

