
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |The article describes the influence of the material features and critical process parameters inside a fully Continuous Direct Compression plant.
Evaluation of critical mixing parameters in a Continuous Direct Compression process.
The following article describes the influence of the material features and critical process parameters inside a full continuous direct compression plant. A quality by design (QbD) approach was set up to evaluate the critical quality attributes of the tablet press, final blend, and tablet characteristics in a continuous direct compression process. The experimental design consisted of two distinct types of direct compression-grade lactose, SuperTab®22AN and SuperTab®30GR, which were blended with different percentages of lubricant and tracer. This study confirmed that direct compression (DC)-grade excipients facilitate continuous processes by guaranteeing stable blends. This study showed the importance of understanding the characteristics of pure excipients in predicting their behaviour inside a continuous line. Finally, it shows which of the selected process parameters is critical and affects the quality of the final product, which is the most relevant for optimizing the process.
Introduction
The concept of Pharma 4.0 is strictly connected to innovations, improvements, and an enhanced gap from traditional approaches. Pharmaceutical companies and supplier manufacturers are undergoing a transition from traditional batchwise manufacturing to Continuous Manufacturing. Economic, technological, and regulatory challenges associated with current pharmaceutical manufacturing methodologies have encouraged the industry to invest in more efficient and reliable product manufacturing technologies such as Continuous Manufacturing.[1]
This definition enables us to understand why this approach differs. In a batch process, raw materials are charged into a predefined system at the beginning of the process, and the product is discharged and collected at the end of the process. No ingredients cross the system boundaries between the time the raw materials are charged, and the time the final product is discharged. In Continuous Manufacturing, materials and products are continuously charged into and discharged from the system, respectively, throughout the process.[2] This perspective is driven by several advantages of continuous processes, such as improved efficiency and product quality, reduced manufacturing costs, and increased flexibility for large-scale production.[3]
It is well known [4] that tablets, among oral solid dosage (OSD) forms, are used most because of their advantages such as stability and low-cost production processes. Direct compression (DC) is the desired approach to save money and enhances line efficiency. The already long-existing continuous DC technology has been extensively studied over the past few decades. However, tableting in DC mode requires strong knowledge and research to choose suitable active pharmaceutical ingredients (API) and excipients. Despite the continuous character of DC, it is generally operated in a semi-continuous mode because the blends used in DC are made in batch mode. The combination of Continuous Manufacturing and direct compression, so-called continuous direct compression (CDC), represents a high-degree challenge for machine manufacturers, powder suppliers, and pharmaceutical companies, mainly because of poor intellectual property incentives, lack of proven equipment,[5] and existing formulations for batch technology approaches.
Because of its advantages,[1] CDC technology adoption is encouraged at economic, technological, and regulatory levels. This article aims to explore with a quality by design (QbD) approach the impact of critical process parameters (CPPs) on the critical quality attributes (CQAs) in a CDC process.
Materials and methods
Materials
The formulations were prepared using fillers, tracers (as a model), and lubricant. Two distinct types of direct compression-grade lactose were selected as fillers: SuperTab® 22AN and SuperTab® 30GR, both manufactured by and obtained from DFE Pharma GmbH & Co KG, Germany. SuperTab® 22AN is an anhydrous grade lactose with low water content and no crystal water. SuperTab®30GR is an agglomerated lactose consisting of lactose monohydrate containing almost 5% crystal water. The free water content of both grades was extremely low (< 1 %). The API model (tracer) used was FD&C Blue nr. 2 dye (indigo carmine Lake, Colorcon, PA, USA). The resulting blends can be considered adhesive mixtures because of their fine particle size (99.9%>US mesh 325, which corresponds to an x99 far below 45 µm) and low tracer content (1 wt. %) in the blend. Finally, magnesium stearate (Ligamed MF-2-V, Peter Greven, Venlo, The Netherlands) was selected as the lubricant to facilitate tableting.
Continuous Line setup
A fully Continuous Direct Compression manufacturing line was used (Figure 1).
![]()
Figure 1: Continuous direct compression line, IMA, Ozzano dell’Emilia, Bologna, Italy.
The materials (excipients, API model, and lubricant) were fed into the continuous horizontal mixer from two distinct positions using three different loss-in-weight feeders. The blend flowed towards the rotary tablet press placed downstream of the continuous mixer. A near infrared (NIR) spectroscopy sensor (MicroNIR® PAT-U, VIAVI Solution) was installed as the process analytical technology (PAT) unit. This allowed for control of the blend uniformity of the future tablet owing to its positioning immediately before the powder was loaded into the tableting die (Figure 2).

Figure 2: MicroNIR PAT-W installed on Prexima 300 feeder.
The Gericke Formulation Skid (GFS) was used for these trials [6], and a Prexima 300 rotary tablet press was selected from the Prexima family because of its throughput capability that is compatible with the upstream setup.
Trial setup
The methodology used for these trials was structured and organized according to the ICH Q8 (R2) guidelines for pharmaceutical development [7]. For this reason, the design of experiment (DoE) method was selected [8] to study the mixture characteristics (MCs) and critical process parameters (CPPs) (Table 1).
| Mixture | Variable type | Range | Type |
| % Lactose | Mixture | 96% – 98% w/w | Continuous (Sum 99%) |
| % MgSt | Mixture | 1% – 3% w/w | Continuous (Sum 99%) |
| Lactose Type | Mixture | 30 GR – 22 AN | Categoric |
| Impeller Speed | Process | 60 RPM – 180 RPM | Continuous |
| Weir Type | Process | 1 – 2 | Categoric |
| MgSt Inlet | Process | Point 2 – Point 3 | Categoric |
Table 1: Material characteristics and critical process parameters selected for the continuous direct compression process.
To evaluate how the variations in MCs and CPPs affect the process, several numerical responses were identified, the so-called critical quality attributes (CQAs). The CQAs were selected at three levels in the process: first, blend consistency (percentage of tracer and its relative standard deviation (RSD) and homogeneity through NIR probe – moving block mean, moving block standard deviation, and moving block RSD); second, tablet press responses (dosage chamber – automatically adjusted to always have the same tablet weight, pre compression force, and its RSD); and third, tablet characteristics (tensile strength, percentage of tracer, and RSD).
Near-Infrared Spectroscopy
As mentioned in the previous paragraphs, the Micro-NIR sensor developed by VIAVI was installed inside the feed frame to collect the spectra of the powder that was about to enter the tablet dies. The acquisition of spectra was automated to perform an online moving-block (MB) analysis. The moving-block algorithm was applied to observe whether some clear variations allowed the operator to understand that the process was shifting out of control. This involves summarizing the variance in the blocks of spectra collected over the time course of the process. Two pre-treatments of the data were used: the first derivative (11 pt., 2nd order polynomial) and the standard normal variate. Once the trials were performed, all collected spectra were further analysed using the Unscrambler X® Software v11 (Camo Analytics).
Tracer content uniformity of powder blends and tablets
The FD&C-2 Blue Lake content in powder and tablet samples was determined by UV-VIS analysis. This was done by accurately dissolving 250-300 mg of a powder sample or one tablet of approximately 300 mg, accurately weighed, in 100 ml of borate buffer solution (0.1 M), adjusted to pH 9.10 with sodium hydroxide. The complete dissolution of the samples was confirmed by visual observation, followed by filtration of the sample with a 0.45 µm syringe filter to remove the undissolved magnesium stearate. The dye content was quantified by UV-VIS analysis on a UV-VIS spectrophotometer (Lambda 25, Perkin Elmer, MA, USA) in 1.00 cm disposable PMMA cuvettes at a wavelength of 611 nm. The dye content was calculated using a calibration curve obtained by analysing the dilution range of a solution of FD&C-2 Blue Lake in the same borate buffer. Lactose was added to the calibration solution at a concentration of 99 wt. % relative to the Lake to obtain a comparable solution to the actual sample solutions. To determine the content uniformity of the powder samples, 15 distinct samples with a volumetric sample scoop were obtained at various locations in the powder samples, as described previously [2]. Content uniformity was determined as the RSD from the measured content values for 15 samples of the powder blend or 10 tablets.
Results and Discussion
Materials
The particle size distributions (PSD) obtained by laser diffraction of SuperTab®22AN and SuperTab®30GR are shown in Figure 3 and Table 2.
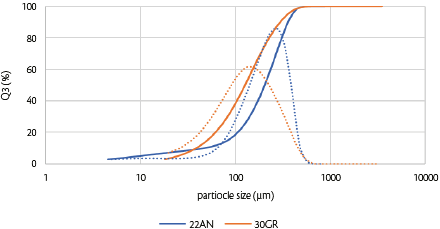
Figure 3: Particle size density and cumulative distributions by laser diffraction of direct compression grade lactose (SuperTab® 22AN and SuperTab® 30GR).
| Mixture | X10 µm |
X50 µm |
X90 (µm) |
Span (-) |
BD (g/ml) |
TD (g/ml) |
HR (-) |
True density (g/ml) |
| SuperTab®22AN | 47 | 203 | 359 | 1.54 | 0.64 | 0.82 | 1.28 | 1.58 |
| SuperTab®30GR | 38 | 126 | 297 | 2.05 | 0.63 | 0.78 | 1.24 | 1.53 |
Table 2: Physical characteristics of SuperTab®22AN and SuperTab®30GR: PSD (x10, x50, x90, span), density data (poured bulk density (BD), tapped bulk density (TD), Hausner ratio (HR)), and apparent true density.
These two materials are the major components of powder blends and tablets (96-98 wt. %) and determine blend properties such as powder flow and tabletability.
The two types of DC-grade lactose had PSDs; however, there were some differences. SuperTab®22AN had a broader particle size distribution, which was reflected in the calculated span, and SuperTab®30GR had a lower tapped bulk density, lower true density, and better flowability, reflected in the lower Hausner ratio (HR, Table 2). Span was calculated from the PSD (Equation 1),
![]()
whereas the HR was calculated from the poured (BD) and tapped bulk density (TD) (Equation 2).
![]()
The differences in morphology and particle size were clear from the SEM images (Figure 4).
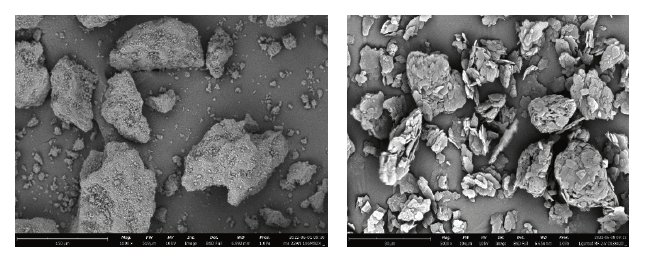
Figure 4: Scanning electron microscopy (SEM) images of SuperTab® 22AN (left) and SuperTab® 30GR (right) types of direct compression (DC)-grade lactose’s at 1000x magnification.
SuperTab®22AN consists of large solid particles with few pores. In contrast, SuperTab® 30GR consists of particles that are composed of many smaller particles (agglomerates), and as a result, it has more pores. The fine fraction in SuperTab®22AN was distributed on the surface of the particles, as already demonstrated [2].
These clear morphological differences have a significant effect on blending and compaction. Pores can accommodate small particles such as magnesium stearate and micronized compounds such as active pharmaceutical ingredients (APIs) or, in this study, micronized tracer compounds (Figure 5).
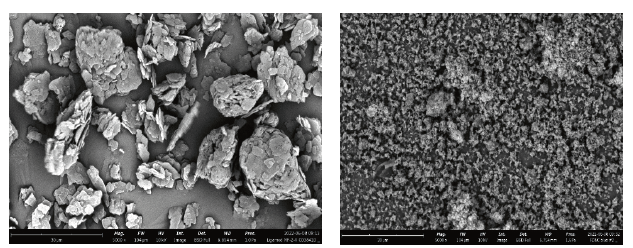
Figure 5: SEM images of Left: magnesium stearate (Ligamed MF-2-V) and right: FD&C blue #2, both at 5000x magnification.
The morphology of magnesium stearate (Figure 5) was completely different. It consists of small particles built from thin plates. These plates can be spread on the surface of excipient particles, and prolonged blending will facilitate this effect and negatively influence tabletability.
Process Parameters
The effect of the different densities of both types of lactose was reflected in the height of the dosing chamber.
This is selected to provide the tablet weight and represents the distance from the die plate to the lower punch. The average dosage size was only slightly dependent on magnesium stearate content (Figure 6) whereas the type of lactose had a significant effect: an average of 6.3±0.1 mm for SuperTab®22AN versus an average of 7.2±0.1 mm for SuperTab®30GR that has a lower bulk density (Table 2).
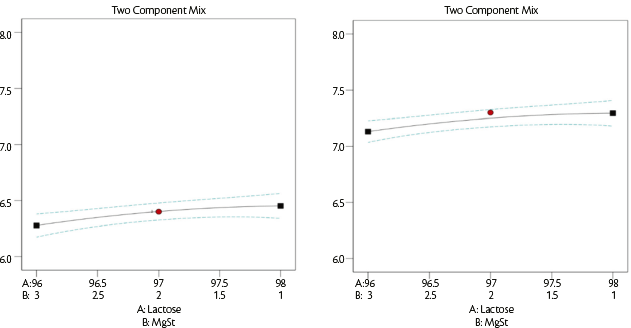
Figure 6: Dosing chamber [mm] vs lubricant quantity for SuperTab® 22AN (on the left) and SuperTab® 30GR (on the right).
The impeller speed of the continuous mixer did not have a considerable influence on the responses. The lubricant inlet port slightly affects the compaction force. The port closest to the exit of the axial mixer, port 3, resulted in an enhancement of the pre-compression force (PCF, Figure 7) caused by the lower lubricant residence time inside the mixing chamber. This effect was even more pronounced when weir 2 was installed: the geometrical configuration of this weir allowed for a reduced residence time and hold-up with reduced lubricating efficiency of the particles, and hence an increased pre-compression force (PCF) as the result (Figure 7).

Figure 7: Pre-Compression Force (PCF) [kN] as a function of weir type and magnesium stearate (MgSt) inlet port at 1% MgSt (left) and 3% MgSt (right) with SuperTab® 22AN.
The level of lubrication and the lubricant inlet port are the most important process parameters in determining the tablet tensile strength. The insertion of magnesium stearate via the inlet at port 2 (farther from the mixer exit) caused a reduction in the tensile strength of the tablets with increasing lubricant content (Figure 8).
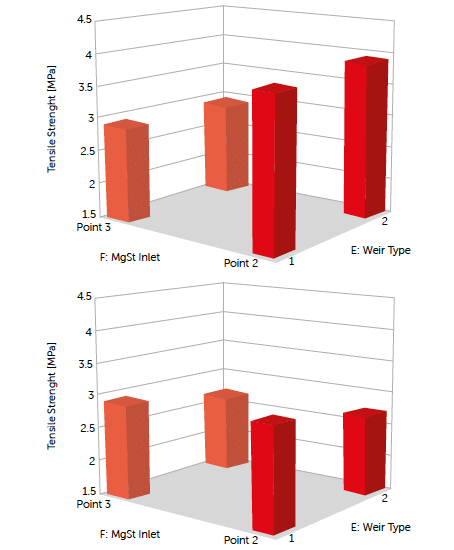
Figure 8: tablet tensile strength (TS) [MPa] as a function of weir type and magnesium stearate (MgSt) inlet port at 1% MgSt (left) and 3% MgSt (right) using SuperTab®22AN as a filler.
This reduction was found for both grades of DC lactose, but not at the same level. The reduction in tensile strength for SuperTab®30GR was lower than that for SuperTab®22AN. Feeding magnesium stearate via port 3 (closest to the blender exit) showed a similar trend in lubricant sensitivity, but with a smaller difference owing to the reduced residence time of the lubricant.
Process Stability
Process stability was monitored online by NIR spectroscopy using moving block analysis. The online moving block was calculated every time a new spectrum was recorded, and the moving block standard deviation (MBSD) and moving block mean (MBM) were evaluated to obtain the behaviour along the entire process to understand immediately if the process was kept inside the design space and if some deviation occurred.
A representative example of MBSB and MBM recording (Figure 9) clearly shows the absence of detectable deviations from homogeneity: the standard deviation (blue line) of the group of spectra collected is close to 0 (highest spike at 0.006%), and the mean (red line) remains constant (discrepancy lower than 0.01) showing that over time, the blends flowing through the tablet press are homogeneous at each point.
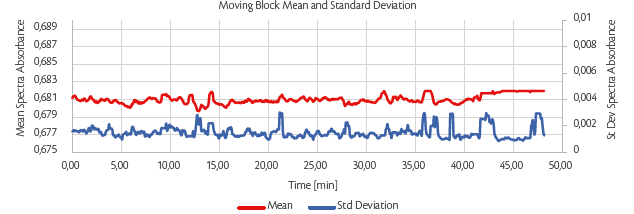
Figure 9: tablet tensile strength (TS) [MPa] as a function of weir type and magnesium stearate (MgSt) inlet port at 1% MgSt (left) and 3% MgSt (right) using SuperTab®22AN as a filler.
The determined tablet features were the tablet weight, tracer content, and content uniformity. These features were stable over time, resulting in a RSD of tablet weight of < 1%. Moreover, no significant correlation was found between the content uniformity of the tracer in the blends and the tablets with process time, ensuring homogeneity of mixing. It was already well known that these excipients exhibit excellent performance in continuous direct compression processes [9]. When mixing was included in the continuous mode, the process in combination with the DC excipients guaranteed excellent content uniformity and process stability even when several process settings were changed.
Conclusions
In this study, a continuous pharmaceutical blending process was tested using various materials and process conditions to evaluate the influence of the downstream process on final product quality. The excipients used in the continuous mode guaranteed a stable process, which was illustrated by a high degree of content uniformity in the tracer content. Tablet characteristics, such as tensile strength, were stable during the process. The only variability in these characteristics was the type of DC lactose and lubricant level used. Therefore, it is important to understand the characteristics and interactions of the excipients used before their application in a continuous direct compression line. The inlet ports are the components most relevant process parameters for an axial blender. The inlet point determines the distance to the outlet and, consequently, the residence time and the number of times the paddles hit the materials during mixing. The impeller speed did not strongly affect the final tablet quality attributes, but at the same time, without the blending tool movement, there was no mixing. This parameter should be optimized as the final parameter to adjust for all desired critical quality attributes. Finally, the weir type had an intermediate influence on the CQAs, owing to its strict correlation with the inclination of the bed powder inside the blender.
In conclusion, process stability was maintained throughout all trials owing to the direct compression grade types of lactose in combination with high dosing precision, flexibility, and capability of the tablet press downstream.
References
1. Moghtadernejad S. et al., A Training on: Continuous Manufacturing (Direct Compaction) of Solid Dose Pharmaceutical Products, “Journal of Pharmaceutical Innovation”, 13:155-187, 2018
2. Jaspers M. et al., Impact of excipients on batch and continuous powder blending, “Powder Technology”, vol. 384, pp. 195-199, 2021.
3. Lee S.L., O’ Connor T.F., Yang X., et al., Modernizing pharmaceutical manufacturing: from batch to continuous production, “J Pharm Innovation”, 10(3):191-199, 2015.
4. Srinath et al., Formulation and Evaluation of effervescent tablet of paracetamol, IJPRD, Vol 3(3), 12 pp. 76-104, May 2011.
5. Evarsti T., et al., Continuous manufacturing of extended-release tables via powder mixing and direct compression, “International Journal of Pharmaceutics”, 495, pp. 290- 301, 2015.
6. Gericke, Equipment for continuous manufacturing of OSD, gerickegroup.com
7. ICH Q8(R2), Pharmaceutical Development Guideline.
8. Politis S.N., Colombo P., Colombo G., Rekkas D.M., Design of Experiments (DoE) in pharmaceutical development, Drug Development and industrial pharmacy, 2017.
9. Hebbink G. A., Dickhoff B. H. J., Application of lactose in the pharmaceutical industry, “Lactose Elsevier”, pp. 175–229, 2019 https://doi.org/10.1016/B978-0-12-811720-0.00005-2


