
Significance of Process Performance Qualification Approach for Process Validation: A Case Study Focusing on the Impact of Process Scale-up on Lyophilizer Pressure Control.
Introduction
The drug product lifecycle concept links product development, process development, and qualification of the commercial manufacturing process to a common process validation paradigm which remain active throughout the lifecycle of the drug product. This lifecycle approach to process validation,
in turn, promotes a development paradigm in the pharmaceutical manufacturing process which focuses on a scientific understanding of the relationship between process variables and product quality attributes1. The cGMP regulations for validation of drug manufacturing requires that drug products be produced with a high degree of assurance, meeting all the attributes they are intended to possess2 and the USFDA approach to process validation involves three stages3 as shown in figure 1.

Figure 1. USFDA three-stage process validation guideline, 2011
The development of a lyophilized drug product starts with a QbD approach which requires a determination of the quality target product profile (QTPP) of the final product at the conceptual stage. Once the QTPP is set, the critical process parameters (CPP) and critical quality attributes (CQAs) are identified to derisk the product development process. Upon conclusion of the QbD process development, the manufacturer must demonstrate that the commercial manufacturing process possesses the desired consistency and robustness to demonstrate the effectiveness of the QbD approach. This is where the second element of the process validation, i.e., qualification of the commercial manufacturing process commences. One approach to evaluate the robustness of the commercial lyophilization process involves challenging the GMP lyophilizer with ‘at-scale’ engineering runs with a scaled-up lyophilization process. Such an approach, which appropriately challenges the validity of the normal operating range (NOR) of the equipment, is considered a Process Performance Qualification (PPQ) run.
The number of PPQ runs for a lyophilized product development may vary depending upon proximity of the GMP lyo process towards the edge of failure. Additionally, a PPQ campaign may involve execution of a formal Failure Mode and Effects Analysis (FMEA) to identify the unknown gaps in process knowledge and risk presented by potential in-process deviations such as transient excursions in pressure or temperature4.
Significance of PPQ in Process Validation Cycle
Contract Manufacturing Organizations (CMOs) that have experience in GMP manufacture of lyophilized products are always in demand and with the advent of the COVID-19 pandemic their demand has skyrocketed5.
Many CMOs collaborate with the IMA process development lab to implement their PPQ campaign in sync with the process validation practices recommended by USFDA and EMEA. With decades of GMP technology transfer experience coupled with the availability of secure internet of things (IoT) remote gateways, the IMA LAB4LIFE process laboratory can provide much of the technical guidance remotely via latest project management platforms.
For most CMOs, the Equipment Qualification (EQ) part of the PQ typically gets concluded during the installation phase. Therefore, the first step in commercialization of a new lyophilized drug product for them is usually a pre-PPQ step that consolidates the CPP and CQAs from the initial QbD development executed in the R&D lyophilizer. Some CMOs, however, may not have an in-depth understanding of the proportional-integral-derivative (PID) control loop required to qualify their GMP lyos for a fit-for-purpose process designed for a new formulation. The case study discussed in this article shows how a migration from a lyo campaign with a crystalline drug product to a new campaign involving a protein-based drug product with 7% trehalose as the bulk stabilizer can result in an unacceptable pressure control in the GMP lyo during full-scale PPQ runs. A team of IMA development scientists performed the root cause analysis (RCA), conducted an impact assessment, and implemented a fit for purpose solution via real-time, remote tuning of the PID control loop for the N2-flow valve. The fit for purpose tuning of N2-flow valve resolved the unacceptable pressure control deviation which stood in the way of commercialization of a very high value protein-based injectable product.
PPQ Runs
Pre-PPQ Preparations and Initial Observations
Upon commencement of a new lyophilization campaign for an amorphous protein placebo formulation, the vial heat transfer coefficient (Kv) tests with water load showed a normal chamber pressure control at 37 microns, 75 microns and 150 microns. With the successful capture of Kv in the GMP lyo; receipt of the QbD scaled up cycle and product CQAs; and passing of CIP, SIP, leak rate check and an ultimate vacuum test; the CMO lyophilization team commenced the full-load PPQ lyophilization runs for the GMP validation process.
Full-Scale PPQ Runs
The at-scale product load was 5.6 L of formulation distributed across 24,000 vials in a 6 + 1 shelved (5.2 m2) GMP lyophilizer with a bottom internal condenser. A representative at-scale lyo process is shown in the following plot (figure 2).

Figure 2. Representative lyo process plot in the GMP lyophilizer.
Identification of Process Deviation During
Full-scale PPQ Runs
As a contracted lyo-SME responsible for supporting the PPQ campaign, IMA LAB4LIFE analyzed the process data collected during the early PPQ runs and made some key observations. The freezing steps of the commercial cycle were uneventful and so was the initial primary drying step.
However, the first full-scale PPQ run resulted in short transient pressure fluctuations from mid-primary drying until just prior to the Pirani drop, typically observed towards the end of primary drying.
Root Cause Analysis (RCA) of the Process Deviation
Upon receipt of the lyo process data with product temperature trends (not shown), IMA development scientists suspected the N2-flow valve to be the culprit although the pressure control was perfect during Kv determination with water load. Presence of the sawtooth pattern of pressure control during the mid-primary drying portion of the lyo process further narrowed down the potential reasons to improper specification of the installed N2-flow valve or its physical damage. The sublimation rate calculation (not shown in this article) confirmed that during early primary drying, the product sublimation rate was sufficient to maintain the chamber pressure setpoint without any N2-bleed valve opening. However, as the sublimation rate during mid-primary drying phase started to drop, the N2-flow valve starts bleeding in the N2 gas to maintain the desired chamber pressure setpoint. At this stage, the PID tuning which worked fine for the previous crystalline drug product and water (another crystalline product) proved inadequate. Therefore, a decision was made by the IMA LAB4LIFE to recommend a set of full-scale PPQ runs with placebo so that PID control of the GMP lyo can be fine-tuned remotely during the full-scale PPQ run.

Figure 3a (Left). The MKS pressure control observed during the primary drying step under the full placebo load.
Figure 3b (Right). The same GMP lyophilization process under full load after PID tuning performed by IMA development scientist.
Basics of PID Control Loop
A control loop is a feedback mechanism that attempts to correct discrepancies between a process variable (PV) and the setpoint punched into the system.
For industrial applications, a PID controller tracks the error between the PV and the setpoint, the integral of recent errors, and the derivative of the error signal. t computes its next corrective effort from a weighted sum of those three terms, applies the results to the process, and repeats this measure-decide-actuate closed loop until the error is eliminated6.
It is noteworthy that every IMA lyophilizer undergoes a Factory Acceptance Test (FAT) with default PID settings but the product specific PID tuning can be, and many times is requested by the CMOs for their respective GMP environment. The PID loop tuning itself is a careful art of selecting values for tuning parameters (P, I and D) that enable the controller to eliminate the error quickly with minimum process variable fluctuations. With regards to drying process evaluation and PID tuning of GMP lyophilizers, the IMA LAB4LIFE was able to provide its expertise given the decades of unparalleled experience in this field.
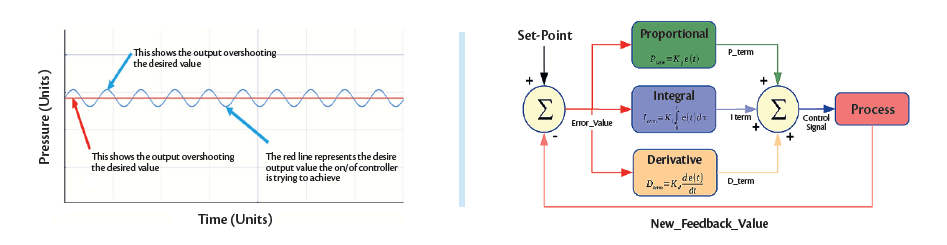
Figure 4a (Left). A representative plot showing the difference between the process setpoint and the process variable (PV).
Figure 4b (Right). A simplified PID control loop schematic showing how a process setpoint is maintained in a modern equipment with the constant com-bined input of P, I and D elements6
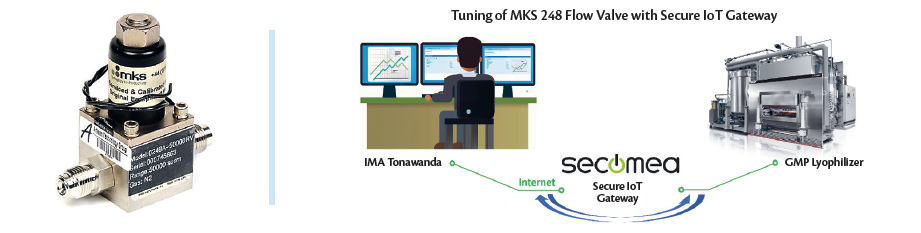
Figure 5a (Left). A MKS 248 flow valve installed in IMA lyophilizers.
Figure 5b (Right). A simple schematic showing the fully- secure, remote access capability available with IMA group to access GMP lyophilizers via SECOMEA gateway.
Impact of Transient Pressure Excursions on Product Quality
The observation of transient pressure deviations during the full-scale PPQ run resulted in the drug product manufacturer requesting IMA Life to analyze the impact of such a process deviation on the
final quality of the lyophilized drug product. Using the Kv information captured during QbD process development and the steady-state heat and mass transfer equations shown below, the IMA LAB4LIFE analyzed the impact of these transient pressure excursions on product temperature and, therefore, the final product quality.
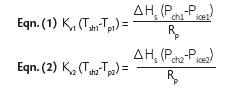
The underlying assumptions made by the IMA LAB4LIFE for the impact analysis are as follows:
- The product resistance to mass flow (Rp) inputs were taken from the primary drying stage of a sucrose lyophilization cycle.
- The Rp does not readily change during a transient pressure excursion event.
- Quasi-steady state conditions were assumed for the calculations.
- The collapse temperature (Tc) for the formulation was accurately captured from Freeze Drying Microscope (FDM) experiments executed as part of the early QbD lyo development efforts.
- The product temperature profiles were selected from the full-scale PPQ run executed in the GMP dryer.
It is important to note that the transient pressure excursions observed during current PPQ runs could have been dismissed to have no or minimal impact on the product temperature due to thermal inertia observed during the primary drying step of the lyophilization process.8 However, IMA LAB4LIFE strongly feels that it was prudent on part of the drug product company to have it analyze the impact
of such process deviations on the product quality in the current regulatory landscape.
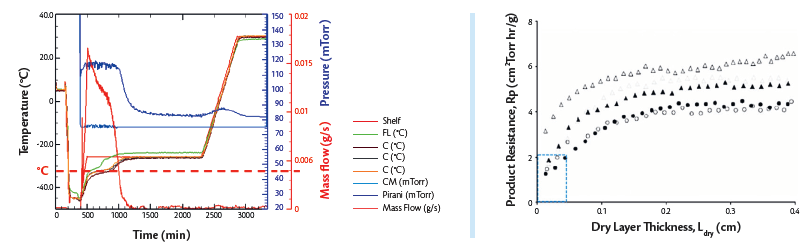
Figure 6a (Left). A representative PPQ process plot showing product temperature profiles used in calculating the impact of the pressure deviation on product quality.
Figure 6b (Right). Change in Rp during Primary drying. Closed and open triangles are for 4- and 2-mL fills, respectively, of 10% sucrose, and closed and open circles are for 4- and 2-mL fills, respectively, of 5% sucrose7.
Summary
One of the essential requirements of a successful process validation strategy is completion of a comprehensive PPQ report where data, results, and analyses are clearly explained along with the assessment of adherence to the PPQ protocol. Additionally, any process deviations and unanticipated results must be described and clearly explained. Finally, a statement indicating whether the lyophilization process delivers the desired product quality and uniformity via product temperature mapping and extended sampling/ CQA testing is essential for the conclusion of the PPQ phase
of a lyophilized drug development campaign. While the product temperature response to such process deviations cannot be fully quantified by quasi-steady-state heat and mass transfer modeling8, the IMA LAB4LIFE ’s impact assessment of such transient pressure excursions focused on extreme scenarios where the primary drying process runs very close to the edge of the failure and the product quality is sensitive to minor process deviations. Such pressure excursions are not uncommon in the lyophilization industry and investigation of such deviations requires an impact analysis which in turn decides the resulting fate of the affected batch.9 At its core, the central question addressed by the IMA LAB4LIFE quantified the impact of pressure deviations on the product temperature.
References
[1] Dolgin, D., Epp, K., Zwolenski-Lambert, W., Rooney, M., Huang, L., & McMenamin, M. (2014, August 1). Lifecycle approach to biotech process validation. ISPE.
Retrieved December 10, 2021. [2] United States Code of Federal regulations, 21 CFR 211.100 (a) and CFR 211.110 (a). [3] FDA. (2011, January). Process validation: General principles and practices. Retrieved December 10, 2021. [4] Ammoscato, V., & Stankovic, C. J. (2016, September 29). Process performance qualification prep: A strategic approach leads to success. American Pharmaceutical Review. Retrieved December 10, 2021, [5] Grand Stanton, D. (2021, October 8). CDMOs likely to continue to ride COVID-19 wave. BioProcess International. Retrieved December 10, 2021. [6] Vandoren, V. (2016, July 26). Understanding PID control and loop tuning fundamentals. Control Engineering. Retrieved December 10, 2021. [7] Patel, S. M., Bhugra, C., & Pikal, M. J. (2009). Reduced pressure ice fog technique for controlled ice nucleation during freeze-drying.
AAPS PharmSciTech, 10(4): 1406. https://doi.org/10.1208/s12249-009-9338-7 [8] Adhikari, N., Zhu, T., Jameel, F., Tharp, T., Shang, S., & Alexeenko, A. (2020). Sensitivity study to assess the robustness of primary drying process in pharmaceutical lyophilization. Journal of Pharmaceutical Sciences, 109(2), 1043–1049. https://doi.org/10.1016/j.xphs.2019.10.012 [9] Nail, S., Tchessalov, S., Shalaev, E., Ganguly, A., Renzi, E., Dimarco, F., Wegiel, L., Ferris, S., Kessler, W., Pikal, M., Sacha, G., Alexeenko, A., Thompson, T. N., Reiter, C.,
Searles, J., & Coiteux, P. (2017). Recommended best practices for process monitoring instrumentation in pharmaceutical freeze drying – 2017.
AAPS PharmSciTech, 18(7), 2379–2393. https://doi.org/10.1208/s12249-017-0733-1

