
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |VERISEQ® Nucleation: Better Control of Nucleation in Lyophilization
The lyophilization process consists of three phases: freezing, sublimation, and desorption. The freezing phase impacts the crystal structure that influences the drying rate and contributes to batch uniformity.
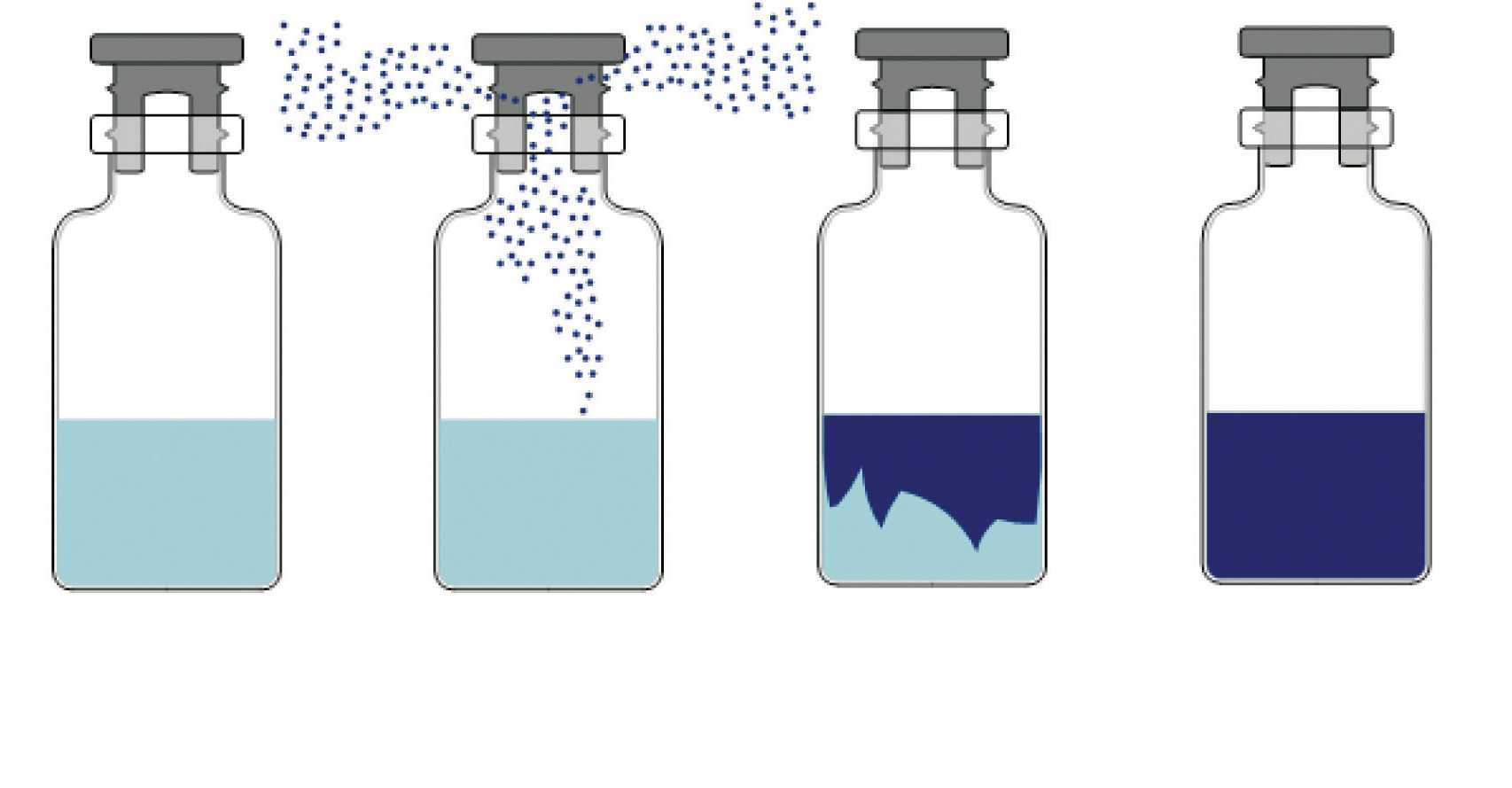
In sterile lyophilization, liquid-loaded vials can nucleate over a range of time with different degrees of supercooling, resulting in inter-batch varied ice crystal sizes which impact product resistance to drying.
VERISEQ® Nucleation is a controlled nucleation technology capable of producing sterile cryogenic ice fog and circulating it within a lyophilizer chamber to ensure reliable and prompt nucleation of vial contained pharmaceutical formulations.
As a result, vial to vial variance is reduced, ice crystals are larger, and control of the lyophilization process is enhanced.
In a recent application in India, the lab-scale VERISEQ® controlled nucleation system was installed for a pharmaceutical product which has critical residual moisture and batch uniformity requirements. The controlled nucleation system was retrofit to a lyophilizer at the India facility. During onsite startup, full nucleation was achieved with 2ml, 5ml, 10 ml, 20ml, and 100ml vials.

