Prexima and Croma, integrated technologies for Continuous Manufacturing.
Jola Gorreja, R&D Process Engineer at IMA Active Guia Bertuzzi, Product Manager for process equipment at IMA Active |
Welcome to the future, the new world where production goes by the name integration.
Where a single system manages transformation from powder to tablet. This is the new pharmaceutical production frontier, where Prexima and Croma are one.
Presented at Achema 2018, Croma is IMA Active’s first step towards a more comprehensive vision of Continuous Manufacturing.
Croma is designed to work in a truly continuous mode. Product is flowing continuously throughout the modules, with no steps. The breakthrough of Croma lies into the product movement. Both the design of the drum, the position and shape of the mixing baffles provide motion to the minimum unit of product, thus keeping its flowing under control. Modularity is the hallmark of the project. Croma can be fitted with up to four coating modules, that can be connected either in series for higher weight gain or in parallel for higher throughput. Each module can be adjusted to work with different process parameters and can perform different processes, if required. The result is the maximum flexibility in terms of machine configuration and process performance.

Croma is fitted with highly innovative technologies for process monitoring and control to deliver coated tablets with specific consistent properties. Real-time monitoring employs a combination of process parameter trends, measurements by PAT tools and sources of process analytical data that can facilitate decision making and follow-up action.
This article highlights how Croma and Prexima 300 are sized to work within a Continuous Manufacturing line, showing all the possible architectures of the system. An integrated control system is required to make the whole process work according to a Quality by Design approach.

The new pharmaceutical production frontier, where Prexima and Croma are one
A pharmaceutical tablet must satisfy certain standards to be considered as a high quality drug. These specifications regard appearance, uniform weight and thickness, hardness, friability, disintegration and dissolution time, API content uniformity. Some of these properties change over time due to the aging process of tablets. As a matter of fact, tablet aging is affected by API characteristics, formulation and lubrication, manufacturing process variables and storage conditions, mainly related to humidity and temperature. Tablet aging can start after the compression process due to a deformation mechanism called “stress relaxation”.
When the in-line connection between a tablet press and a continuous coater is requested, it is necessary to consider the materials characteristics to be processed. One example for all: if the tablets are very viscoelastic, it is not possible to coat them immediately, otherwise the stress relaxation can drastically affect the coating process, with possibility to break the coating layer in the worst case. For this reason, it is extremely important to find solutions to manage these tablets changes according to the relaxation time.
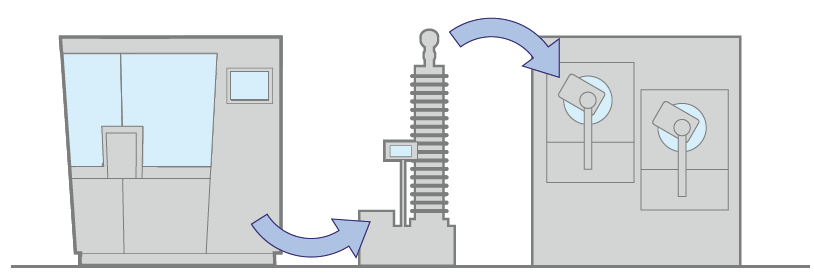
1. Without buffer, in the same room
When the relaxation time is practically zero, it is possible to have this first configuration. In this case, the process is truly continuous and the tablet friction stress is reduced to a minimum.
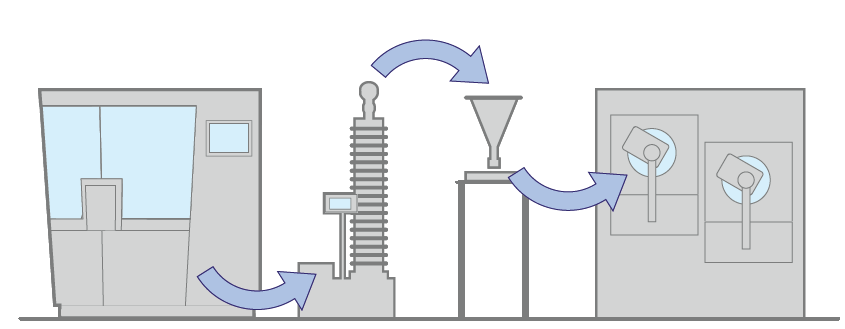
2. With single buffer, in the same room
When the relaxation time is in the order of minutes, this second configuration becomes the best choice. In this case, the buffer is used for helping the tablets to reach the stability condition. The buffer can also compensate the stop condition of one machine – for example it is possible to keep the coater running while the tablet press is in stand-by for limited period.
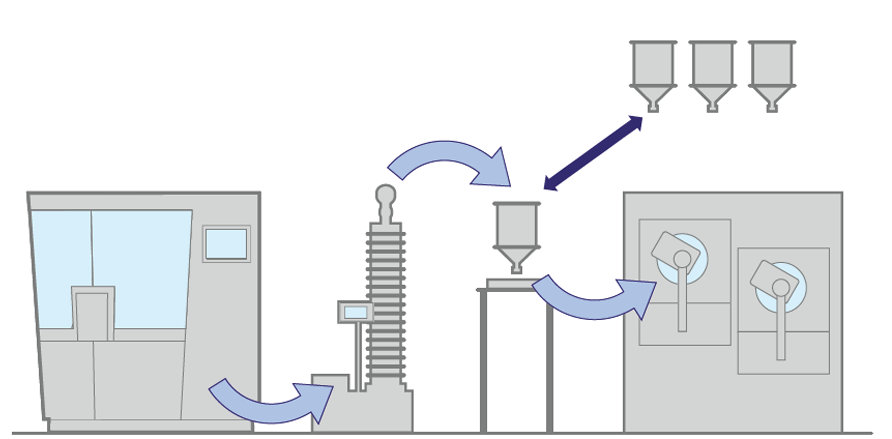
3. With multiple buffer, in the same room
When the relaxation time is in the order of hours, the buffer must have a bigger volume in order to manage the complete process. A possible solution to increase this volume could be the one with multiple buffers equipped with an automatic switching device.
As a general conclusion, manufacturing optimization depends on tablet and coating formulations, process parameters and equipment configuration. Analyzing all these variables and understanding their effects is the key to obtain a perfect continuous process.
Last submitted Papers
- The Prexima challenge. Comparison of rotary tablet presses seminar in Leverkusen (Germany), 2-4 July 2019.
- How to perform a good scale-up
- Development of an automated multi-stage continuous reactive crystallization system with inline PATs for high viscosity process
- Prexima 300. Determination of the effect of the pre-compression force on the tablet hardness, obtained at constant value of the main compression force
- FMECA Risk Analysis background for calibrated containment solutions
