
Quick access to IMA Sectors:
Pharma | Food & Dairy | Confectionery | Tea & Beverage | Coffee | Personal & Home Care | Tissue & Nonwoven | Automation | Tobacco | E-Commerce |Manufacturing DPIs: an engineering perspective

1. Abstract
When developing new pharmaceutical products in DPI form, industrial manufacturing aspects must be considered from the very beginning to shorten the scale-up and optimization of the final manufacturing process, as well as to achieve a more efficient and cost effective production. Precise micro-dosing, weight control, containment measures and ease of device assembly are all issues that must be faced at an early stage.
IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and assembly. Gravimetric weight control performed in line on each single capsule or device, both before and after filling. Absence of mechanical powder compression for improved airway intake. Accurate micro-dosing and automatic feedback and adjustment. Highly flexible and precise inhaler assembly in accordance with design for manufacturing (DFM) and proof of principle studies at an early stage.
This article investigates optimal process parameters for low-dose DPIs achieved by the dosator technology.
The study proves that a major advantage of using this technology for processing DPIs is that the dosators can be accurately adjusted without any need to compress or aspirate the powder. Maintaining the free-flowing properties of the dispended powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced; thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.
2. Introduction
In 1948, the first commercial dry powder inhalation device was launched on the market. This first technology seems archaic by today’s standards: a deep inward breath would cause a ball to strike a cartridge containing powder and shake the powder into the airstream. Since then, changes in the drug delivery market and regulatory pressures have driven innovation of DPIs forward. Firstly, the introduction of capsules has meant standardised filling technologies can be incorporated into the manufacturing process, thus meeting the needs of large-scale industrial filling of such devices. With the availability of accurate filling technologies, it is possible to manufacture DPIs on a large scale to meet worldwide volume needs at acceptable costs. Afterwards, the 1987 Montreal Protocol, which called for minimising the chlorofluorocarbons (CFCs), diverted market interest away from CFC-propelled Metered Dose Inhalers (MDIs) to DPIs. In the end, healthcare reforms in fast-growing economies did the rest. The availability of low-cost, patient-friendly DPI options encouraged their use also in Asia and Latin America, where MDIs are often still preferred because considered more cost-effective. It is estimated by the WHO that, worldwide, some 300 million people suffer from asthma and 240 million people suffer from chronic obstructive disease (COPD). DPIs represent 50% of the total asthma/COPD market by value worldwide. The latest patient-focused studies using DPIs indicated that the expectations regarding this technology have evolved. Patients and pneumologists are now increasingly focusing on convenience and ease of use, favouring a compact design. Indeed, DPIs have shown great promise in their ability to deliver drugs reliably and effectively, and novel designs can ensure that future cost, compliance and safety challenges are overcome. Some of the performance characteristics essential to DPIs are related to dose delivery, fine particle fraction content and performance levels at varying airflows. These characteristics can differ from one powder formulation to another, and some fine tuning of either device or formulation or a combination of both may be necessary to achieve optimal performance. Micro-dosing DPIs takes this challenge to extremes. IMA draws on its extensive expertise to provide the most advanced solutions for DPI processing and assembly.
3. Case study: investigating optimal process parameters for low-dose Dry Powder Inhalers
Aim of the study was to explore the best process parameters to achieve the 5.5 mg dose of a powder mix including a first Lactose type as carrier and a second one (4% in concentration) of micro fine lactose as API simulator. The process was carried out as a first approach in a table-top capsule filling device (Minima, IMA) and then up scaled to an industrial production scale capsule filling machine (Adapta with 100% gravimetric fill weight control, IMA). Two types of Lactose were compared from different suppliers: Inhalac 251 (Meggle, Germany) and Respitose SV003 (DFE, Germany). In table n. 1 some technological characteristics of the two kinds of powder mixes.
| Powder mix |
Bulk density |
Tapped density (g/ml) |
Carr index (%) |
Loss on drying (%) |
| Inhalac 251 +4% lactose microfine |
0.593 | 0.780 | 23.9 (poor flowability) |
0.04 |
| Respitose SV003 +4% lactose microfine |
0.658 | 0.812 | 18.9 (fairly good flowability) |
0.08 |
Table 1: Inhalac 251 (Meggle, Germany), Respitose SV003 (DFE, Germany).
The target dosage of 5.5 mg was achieved step by step after a preliminary work starting from 25 mg and than 15 mg with both formulations on the Minima machine. In tables n. 2 and n. 3 the results of this first screening is reported including machine setting, net weight achieved, tolerances, range between minimum and maximum sample weight obtained and relative standard deviation.
| Average net weight (mg) |
Doser internal diameter (mm) |
Min-max weight sample deviation (mg)* |
Tolerance obtained |
Relative standard deviation (%)* |
| 25.9 | 3.0 | 1.48 | +3.3/-2.3 | 2.03 |
| 14.6 | 2.5 | 0.74 | +1.6/-3.4 | 1.48 |
| 5.4 | 2.0 | 0.70 | +8.0/-6.1 | 3.0 |
| *Values calculated over the gravimetric fill weight of the 100% of processed capsules. | ||||
Table 2: Inhalac 251+ 4% Lactochem microfine lactose, Minima trials.
| Average net weight (mg) |
Doser internal diameter (mm) |
Min-max weight sample deviation (mg)* |
Tolerance obtained (%)* | Relative standard deviation (%)* |
| 25.5 | 3.0 | 1.75 | +3.4/-3.4 | 2.24 |
| 14.6 | 2.5 | 0.46 | +1.5/-1.6 | 0.90 |
| 5.5 | 2.0 | 0.67 | +4.8/-8.1 | 2.9 |
| * Values calculated over the gravimetric fill weight of the 100% of processed capsules. | ||||
Table 3: Respitose SV003 + 4% Lactochem microfine lactose, Minima trials.
It was demonstrated that both formulations gave good results in terms of machinability and tolerance obtained. No significant difference was observed by the operator.
The second step of the study was to up scale the experience gained on the bench top machine to the production equipment. Since the target dose was 5,5 mg the main work was concentrated on this target with both preparations.
Again both formulations demonstrated good behaviour in the machine without any particular problem (no seizing, no empty capsules produced). This can be also seen in the final results that are summarized in the final table n. 4 below: the powder mixes machine setting are reported including net weight achieved and relative standard deviation for an easy evaluation.
| Lactose kind | Average net weight (mg) |
Doser internal diameter (mm) |
Relative standard deviation (%) |
Machine speed (caps/h) |
| Inhalac 251 +4% Inhalac 500 |
5.5 | 2.0 | 2.52 | 85,000 |
| Respitose SV003 +4% Inhalac 500 |
5.5 | 2.0 | 2.52 | 85,000 |
Table 4: Powder mixes Adapta with 100% gravimetric net weight control trials.
As a conclusion, it was confirmed that for 5.5 mg dosing the range between the minimum and maximum weight value in the table-top capsule filler was always lower than 1 mg. The results obtained once formulations were tested in the production scale capsule filling machine were even better: for both formulations the relative standard deviation was confirmed below 3%. The graphs n. 1 and n. 2 under show the net weights of all 24 dosators of the Adapta with 100% gravimetric fill weight control for both powder mixes.
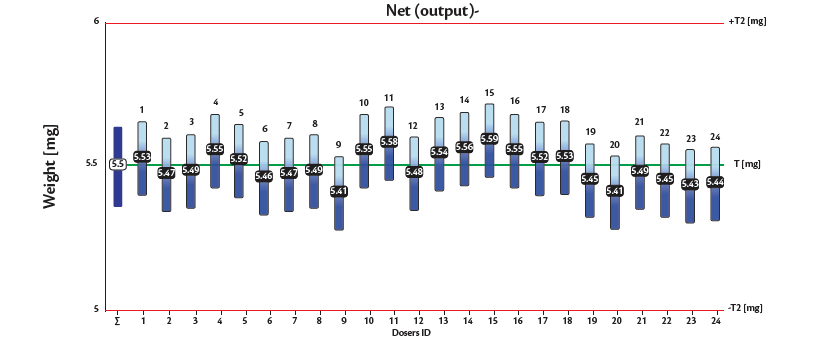
Graph 1: Inhalac 251 + 4% Lactochem microfine lactose, behavior of the 24 dosators on Adapta with 100% gravimetric net weight control.

Graph 2: Respitose SV003 + 4% Lactochem microfine lactose, behavior of the 24 dosators on Adapta with 100% gravimetric net weight control.
As proven by this study, a major advantage of using dosator technology for processing low-dose Dry Powder Inhalers is that the system can dose very small amounts of powders into capsule with maximum precision.
This powder dosing technology does not require powder compaction to transfer the powder to the capsule. This ensures that the powder within the capsule is less likely to form aggregates and is maintained as a free-flowing powder.
Maintaining the free-flowing properties of the dispended powder within the capsule better ensures the release of powder from the capsule into the inhaler when the capsule is pierced, thereby better controlling both the emitted dose and the fine particle fraction of the dose discharged from the DPI.
References
[1] Edwards D, Applications of capsule dosing techniques for use in dry powder inhalers, in “Therapeutic Delivery”, July 2010.
[2] Rogueda P, Take a deep breath. Inhalable drug delivery, in “World Pharmaceuticals”, April 2016.
[3] Williams G, The future of DPIs: Aligning Design with Market Demands, in “Drug Development & Delivery”, December 2012.
